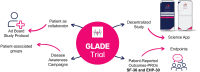
Rationale and design of the GLADE study: a randomized, multicenter, double-blind, placebo-controlled trial evaluating the safety and efficacy of gestrinone subdermal bioabsorbable pellet in endometriosis-related pelvic pain
- PMID: 40641309
- PMCID: PMC12258201
- DOI: 10.1080/07853890.2025.2527352
Rationale and design of the GLADE study: a randomized, multicenter, double-blind, placebo-controlled trial evaluating the safety and efficacy of gestrinone subdermal bioabsorbable pellet in endometriosis-related pelvic pain
Abstract
Background: Pelvic pain secondary to endometriosis is a disabling condition. There are multiple treatments available, with variable endpoints. No prospective controlled studies were carried out evaluating subdermal pellets of gestrinone in this population.
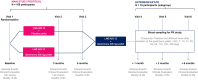
Methods: One hundred participants with documented deep infiltrative endometriosis who underwent surgery without satisfactory response will be randomly assigned (1:1) to either gestrinone 85 mg subdermal bioabsorbable pellets or placebo. Both arms will receive levonorgestrel-releasing intrauterine system (LNG-IUS 12). The treatment duration will be 6 months, with baseline, 3 months and 6 months clinical visits. The primary endpoint is a combination of serious adverse events (SAEs) accumulated within 6 months of insertion of the gestrinone or placebo pellet and collected through spontaneous reports and clinical findings. They include death, threat or risk to life, need for hospitalization, prolongation of pre-existing hospitalization, permanent disability or damage, congenital anomaly; or significant medical occurrences such as venous thromboembolism. The primary safety outcome will be the percentage of patients who do not experience SAEs 6 months after randomization. Androgenization, changes in laboratory exams and in pelvic pain intensity as well as quality of life (SF-36 and EHP-30 questionnaires) will be further evaluated. Daily data on uterine bleeding patterns and the use of pain relief medication will be remotely collected using an App. Pharmacokinetics profile of gestrinone pellet will be characterized.
Conclusion: This is the first multicenter randomized controlled trial to evaluate the safety, tolerability and pharmacokinetics profile of subdermal gestrinone pellets and might inform clinical practice for treating these patients.
Administrative information: GLADE trial is registered at ClinicalTrials.gov (NCT05570786). This is an investigator-initiated research supported by Biòs Farmacêutica.
Keywords: Endometriosis; R-2323; gestrinone; pelvic pain; subcutaneous pellet.
Conflict of interest statement
ER reports grants and consulting fees from Bayer and Pfizer, the Brazilian Ministry of Science and Technology, and personal fees from Aché Pharma, Novartis, and Daiichi-Sankyo outside the submitted work. LBA reports grants from Bayer, Pfizer, and the Brazilian Ministry of Science and Technology. All other authors declare there are no competing interests to declare, and no relevant financial or non-financial competing interests to report.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical