Negative impact of corticosteroid use on outcome in patients with advanced BTCs treated with cisplatin, gemcitabine, and durvalumab: A large real-life worldwide population
- PMID: 40641448
- PMCID: PMC12439076
- DOI: 10.1002/ijc.70009
Negative impact of corticosteroid use on outcome in patients with advanced BTCs treated with cisplatin, gemcitabine, and durvalumab: A large real-life worldwide population
Abstract
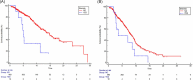
In recent years, there has been increasing interest in the possible prognostic impact of concomitant medications in patients with cancer treated with immunotherapy combinations. This real-world analysis aims to evaluate the impact of concomitant medications on survival outcomes in patients with advanced biliary tract cancer (BTCs) treated with cisplatin, gemcitabine and durvalumab (CGD) therapy. The study cohort included patients with a diagnosis of advanced BTCs who were taking concomitant medications for their comorbidities before the start of CGD. The primary objectives were overall survival (OS) and progression-free survival (PFS). The initial population consisted of 666 patients, who were retrospectively collected from 41 sites in 12 countries. Data on concomitant medications were available for 493 patients. After a median follow-up of 8.8 months (95% CI: 7.8-9.8), patients who did not take steroids (prednisone >10 mg/day or equivalent) or nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, before the start of CGD, had longer OS and PFS in univariate analysis. The multivariate analysis confirmed longer OS for patients who did not take steroids. Patients who did not take steroids had an OS of 14.8 months (95% CI: 13.1-29.1) versus 5.0 months (95% CI: 2.14-11.32) of patients who took prednisone >10 mg/day or equivalent. No differences were reported in terms of overall response rate (ORR), disease control rate (DCR) (p = 1.0 and p = .16, respectively), and safety profile between the two groups. Our analysis suggests that patients who did not receive steroids before the start of GCD had longer survival and highlighted the relevance of balancing concomitant medications and chemoimmunotherapy.
Keywords: biliary tract cancers; cisplatin, gemcitabine, and durvalumab; concomitant medications.
© 2025 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
LR received consulting fees from AbbVie, AstraZeneca, Basilea, Bayer, BMS, Eisai, Elevar Therapeutics, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Jazz Pharmaceuticals, MSD, Nerviano Medical Sciences, Roche, Servier, Taiho Oncology, Zymeworks; lecture fees from AstraZeneca, Bayer, BMS, Guerbet, Incyte, Ipsen, Roche, Servier; travel expenses from AstraZeneca; research grants (to Institution) from AbbVie, Agios, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Jazz Pharmaceuticals, Lilly, MSD, Nerviano Medical Sciences, Roche, Servier, Taiho Oncology, TransThera Sciences, Zymeworks.
ACG reports consulting fees from AstraZeneca, Bayer, BMS, Eisai, Incyte, Ipsen, IQVIA, MSD, Roche, Servier; lecture fees from AstraZeneca, Bayer, BMS, Eisai, Incyte, Ipsen, Roche, Servier; travel expenses from AstraZeneca; research grants (to Institution) from AstraZeneca, Eisai.
SLC serves as an advisory member for AstraZeneca, MSD, Eisai, BMS, Ipsen, and Hengrui, received research funds from MSD, Eisai, Ipsen, SIRTEX, and Zailab, and honoraria from AstraZeneca, Eisai, Roche, Ipsen, and MSD.
TP received consulting fees from Bayer, Ipsen, and AstraZeneca; institutional research funding from Roche, Bayer, and AstraZeneca; travel expenses from Roche.
CB received honoraria as speaker (Astrazeneca, Incyte, Servier) and consultant (Incyte, Servier, Boehringer Ingelheim, Astrazeneca, Tahio, Jazz), received research funds (Avacta, Medannex, Servier) and her spouse is an employee of Astrazeneca.
M. Ikeda reports honoraria from AstraZeneca, Chugai Pharma, Eisai, Incyte, Lilly Japan, MSD, Novartis, Ono Pharmaceutical, Takeda, Teijin Pharma, Nihon Servier, Taiho and research funding from AstraZeneca, Bayer, Bristol‐Myers Squibb, Chiome Bioscience, Chugai, Eisai, Eli Lilly Japan, Delta‐Fly Pharma, Invitae, J‐Pharma, Merck biopharma, Merus N.V., MSD, Novartis, Nihon Servier, Ono, Syneos Health, and Rakuten Medical.
GWP: Advisories and/or Speaker fees: Servier, Bayer, Roche, Amgen, Merck, MSD, BMS, Sanofi, Lilly, Astra Zeneca, Astellas, Pierre‐Fabre, Incyte, Arcus, CECOG.
F. F. has received travel support from Ipsen, AbbVie, AstraZeneca, and speaker's fees from AbbVie, MSD, Ipsen, AstraZeneca.
LP: Advisory role: AstraZeneca, Servier, Travel expenses: AstraZeneca, Ipsen.
GG: Consulting Fees: Astra Zeneca, MSD, Servier, Seagen, Bayer, Amgen, Novartis, Ipsen, BMS.
Travel Expenses: Astra Zeneca, Servier, Bayer, Novartis.
S. Leo reports research funding (to Institution) from Amgen, Astellas, Astra Zeneca, Bayer, Bristol‐Myers Squibb, Daiichi Sankyo, Hutchinson, Incyte, Merck Serono, Mirati, MSD, Pfizer, Roche, Servier; personal honoraria as invited speaker from Amgen, Astra Zeneca, Bristol‐Myers Squibb, Incyte, GSK, Lilly, Merck Serono, MSD, Pierre‐Fabre, Roche, Servier; participation in advisory board for Amgen, Astellas, Astra Zeneca, Bayer, Bristol‐Myers Squibb, Daiichi Sankyo, GSK, Incyte, Lilly, Merck Serono, MSD, Servier, Takeda, Rottapharm.
JD received consulting fees and/or speaker honoraria from Amgen, AstraZeneca, Bayer, BMS, Eisai, Need Inc., Ipsen, Lilly, MediMix, Merck, MSD, Novartis, Roche, and Servier.
JA received consulting fees from AstraZeneca, Jazz Pharmaceuticals, MSD, Roche, Servier, Taiho Oncology, Zymeworks; lecture fees from AstraZeneca, Roche, Servier; travel expenses from AstraZeneca, Roche, Servier.
AD Advisory Board: Amgen, Gentili, Invited Speaker: Eli Lilly, Novartis, Pfizer, Gentili, Amgen, Daiichi‐Sankyo, Roche, Gilead, Travel Support: Eli Lilly, Pfizer, Novartis, Ipsen, Gentili, Gilead, Editorial Collaboration: ACCMED.
GPS received advisory role/travel from Bayer, Roche, J&J, MSD, and Novartis.
MDR received honoraria as a speaker from Astrazeneca.
FC received speaker fees from AstraZeneca, Eisai, OncoSil, Roche, Rovi, and Servier and travel and accommodation from Roche and Servier.
MN received travel expenses from AstraZeneca, speaker honorarium from Accademia della Medicina, Incyte, and Servier; honoraria from Sandoz, Medpoint SRL, Incyte, AstraZeneca, and Servier for editorial collaboration. Consultant; honoraria from EMD Serono, Basilea Pharmaceutica, Incyte, MSD Italia, Servier, AstraZeneca and Taiho.
All remaining authors have declared no conflicts of interest.
Figures
References
-
- Vogel A, Bridgewater J, Edeline J, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow‐up. Ann Oncol. 2022;34(2):127‐140. - PubMed
-
- Rimini M, Puzzoni M, Pedica F, et al. Cholangiocarcinoma: new perspectives for new horizons. Expert Rev Gastroenterol Hepatol. 2021;15(12):1367‐1383. - PubMed
-
- Bertuccio P, Malvezzi M, Carioli G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71(1):104‐114. - PubMed
-
- Lo Prinzi F, Salani F, Rimini M, et al. Efficacy of cisplatin, gemcitabine and durvalumab in patients with advanced biliary tract cancer experiencing early vs late disease relapse after surgery: a large real‐life worldwide population. Oncologist. 2023;12(15):17588359231171574. doi: 10.1177/17588359231171574 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

