Invasive disease-free and overall survival after (neo)adjuvant chemotherapy in postmenopausal patients with hormone receptor-positive, HER2-negative early breast cancer treated with upfront letrozole: Experiences from the phase IV PreFace trial
- PMID: 40641449
- PMCID: PMC12496005
- DOI: 10.1002/ijc.70037
Invasive disease-free and overall survival after (neo)adjuvant chemotherapy in postmenopausal patients with hormone receptor-positive, HER2-negative early breast cancer treated with upfront letrozole: Experiences from the phase IV PreFace trial
Abstract
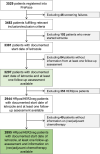
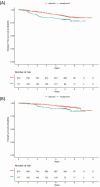
Patients with hormone receptor-positive (HRpos), HER2-negative (HER2neg) breast cancer (BC) benefit less from neoadjuvant chemotherapy (NACT) than patients with triple-negative and HER2-positive BC. In this retrospective analysis of the phase IV PreFace clinical trial (NCT01908556), where postmenopausal HRpos BC patients (n = 3297) were treated with 5-year upfront adjuvant letrozole therapy, we evaluated the prognosis of patients treated with adjuvant versus neoadjuvant chemotherapy in HRpos/HER2neg early-stage BC. HRpos/HER2neg patients with information on (neo)adjuvant chemotherapy (n = 2895) were retrospectively selected from all patients enrolled in the PreFace trial. Invasive disease-free survival (iDFS) and overall survival (OS) were compared between patient groups that were treated with neoadjuvant or adjuvant chemotherapy. Chemotherapy was given to 1051 patients (36.3% of all patients), of which 874 (83.2%) received adjuvant chemotherapy and 177 (16.8%) NACT. Pathologic complete response (pCR) rate in the NACT group was 6.9%. Patients treated with NACT had a worse outcome than those treated with adjuvant chemotherapy (5-year iDFS rate 81% vs. 88%; 5-year OS rate 89% vs. 93%). This effect was maintained after adjusting for age, BMI, lymph node status, grading, tumor size, and histology (hazard ratio for iDFS: 1.95 (95%CI: 1.28-2.95); hazard ratio for OS: 2.13 (95%CI: 1.24-3.66)). Further adjustment for taxane-based regimes did not alter results. In conclusion, in this retrospective analysis of patients with early-stage HRpos/HER2neg BC, patients with NACT had a more unfavorable prognosis than patients treated adjuvantly, independent of patient and tumor characteristics. Prognosis of neoadjuvant patients might be affected by resistance mechanisms, warranting further investigation.
Keywords: adjuvant chemotherapy; breast cancer; hormone receptor‐positive/HER2‐negative; neoadjuvant chemotherapy; phase IV clinical trial.
© 2025 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
P.A.F. received personal fees from Novartis, Pfizer, Daiichi Sankyo, AstraZeneca, Eisai, Merck Sharp & Dohme, Lilly, SeaGen, Roche, Agendia, Gilead, Mylan, Menarini, Veracyte, GuardantHealth, and grants from Biontech, Pfizer, Cepheid, during the conduct of the study; and Translational Research in Oncology (TRIO). C.C.H. received honoraria from AstraZeneca, Daiichi Sankyo, Eisai, Novartis, Pfizer, Roche, Gilead, and MSD, and travel grants from Daiichi Sankyo. B.A. received honoraria from AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Stemline, Teva, Tesaro, Daiichi Sankyo, and Pfizer. S.K. reports personal fees from AstraZeneca, Pfizer, Lilly, Amgen, Hologic, Daiichi Sankyo, MSD Oncology, Sonoscape, Gilead Sciences, Agendia, Roche, Novartis, Exact Sciences, PINK, Celgene, and an uncompensated relationship with WSG. N.N. is currently an employee of AstraZeneca UK Limited and an employee of Novartis Pharma GmbH in the past. C.T. reports being a committee member of AGO, S3‐guideline breast cancer, ESMO, being a co‐editor in chief of BREAST CARE, and receiving lecture/travel fees for lectures at Essener Symposium zur Gynäk. Onkologie und Senologie, Essen, Germany, streamedup! GmbH, Wiesbaden, Germany, Rottalinnkliniken, Eggenfelden, Germany, Universitätsspital Basel, Basel, Switzerland, Onkowissen TV, ESMO congress, Deutsche Gesellschaft für Senologie e.V. H.‐C.K. received honoraria from Pfizer, Novartis, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, TEVA, Theraclion, Janssen‐Cilag, GSK, LIV Pharma, Lilly, Daiichi Sankyo, Gilead, and Zuellig, travel support from Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo, Tesaro, Gilead, AstraZeneca, Zuellig, and Stemline, participated in data safety monitoring or advisory boards for Pfizer, Novartis, SurgVision, Carl Zeiss Meditec, Amgen, Onkowissen, MSD, Gilead, Daiichi Sankyo, Seagen, Genomic Health/Exact Sciences, Agendia, Lilly, and owns stock of Theraclion SA. W.J. has received research grants and/or honoraria from AstraZeneca, Celgene, Chugai, Daiichi Sankyo, Eisai, Exact Sciences, Gilead, GSK, Guardant Health, Janssen, Lilly, Menarini, Stemline, MSD, NeoGenomics, Novartis, Pfizer, Roche, Sanofi‐Aventis, Seagen. A.S. received honoraria from Amgen, AstraZeneca, Aurikamed, Bayer, Celgene, ClinSol GmbH & Co. KG, Clovis Oncology, coma UroGyn, Connectmedica, Daiichi Sankyo, Gilead, GSK, if‐kongress, I‐MED, iOMEDICO, Lilly, MCI Deutschland, med publico, Metaplan, MSD, Mylan, NanoString Technologies, Novartis, onkowissen.de, Pfizer, Pierre Fabre, promedicis, Roche, Seagen, streamedup, SYNLAB, Tesaro, and travel support from AstraZeneca, Celgene, Daiichi Sankyo, Gilead, Pfizer, Roche. M.W.S. received honoraria from AstraZeneca, Pfizer, Clovis, Mylan, Roche, Gedeon Richter, Carl Zeiss Meditec, travel support from Pfizer, and Carl Zeiss Meditec. C.J. reports travel grants and honoraria from Roche, Novartis, Lilly, AstraZeneca, and Exact Sciences. V.M. received speaker honoraria from AstraZeneca, Daiichi Sankyo, Eisai, Pfizer, MSD, Medac, Novartis, Roche, Seagen, Onkowissen, high5 Oncology, Medscape, Gilead, and Pierre Fabre, iMED Institut. Consultancy honoraria: Roche, Pierre Fabre, PINK, ClinSol, Novartis, MSD, Daiichi‐Sankyo, Eisai, Lilly, Seagen, Gilead, Stemline. Institutional research support from Novartis, Roche, Seagen, Genentech, and AstraZeneca. Travel grants from AstraZeneca, Roche, Pfizer, Daiichi Sankyo, and Gilead. C.R. received honoraria from MSD and AstraZeneca, travel expenses from the Swiss Society of Senology and the Swiss Society of Gynecology. P.D. received honoraria from Novartis, MSD, Pierre Fabre, AstraZeneca, Lilly, Gilead Sciences, Daiichi Sankyo, Eisai, and Polytech. E.B. received honoraria from Novartis, Hexal, BMS, Lilly, Pfizer, Roche, MSD, Bayer, Ipsen, Bluebird, B. Braun, and onkowissen.de for consulting, clinical research management, or medical education activities. S.Y.B. has received honoraria from Roche Pharma, Novartis, Pfizer, AstraZeneca, and Teva. T.N.F. has received honoraria from Novartis, Roche, Pfizer, Daiichi Sankyo, and MSD. H.H. received lecture fees from Novartis Pharma GmbH, LEO Pharma GmbH, Atlanta GmbH, and Lilly Deutschland GmbH. C.G. received speaker honoraria from Novartis Pharma GmbH and ClinSol GmbH & Co. KG. K.A. reports personal fees from Roche Pharma AG, Pfizer Pharma GmbH, Seagen, and AstraZeneca. C.W. received honoraria from Lilly, Novartis, Sandoz, Hexal, and Gilead. A.R. received payment for lectures and advisory boards from Roche, Daiichi Sankyo, AstraZeneca, Pfizer, Novartis, Celgene, Exact Sciences, MSD, Pierre Fabre, Lilly, Seagen, Amgen, and GSK. B.R. received research grants from Novartis. All of the remaining authors have declared that they do not have any conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous