Chlorpyrifos induces spermatogenic dysfunction via ferroptosis in Sertoli cells
- PMID: 40641523
- PMCID: PMC12242442
- DOI: 10.1016/j.gendis.2025.101601
Chlorpyrifos induces spermatogenic dysfunction via ferroptosis in Sertoli cells
Abstract
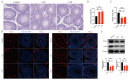
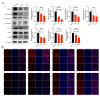
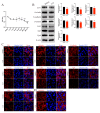
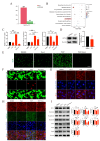
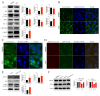
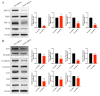
Chlorpyrifos (CPF), a widely used organophosphate pesticide, accumulates in the environment and affects human health. Its neurotoxicity has been extensively studied, and recent research has revealed that it can also lead to abnormal spermatogenesis. However, the factors and molecular mechanisms involved remain unclear. In this study, male Sprague-Dawley rats were gavaged with different concentrations of CPF for 30 days, resulting in a disrupted blood-testis barrier (BTB) and abnormal spermatogenesis. RNA sequencing analysis of Sertoli cells, the primary components of the BTB and key targets of environmental toxins, revealed that ferroptosis-related genes were predominantly among the differentially expressed genes. The expression of ferroptosis-related markers was up-regulated, malondialdehyde and Fe2+ levels were elevated, and glutathione levels were reduced in CPF-exposed testicular tissue and its metabolite TCP-exposed Sertoli cells, confirming that CPF exposure triggered ferroptosis in testes and Sertoli cells. Moreover, treatment with ferrostatin-1, a ferroptosis inhibitor, restored Sertoli cell junctional function. Given the important roles of clockophagy and the HIF-1α pathway in ferroptosis, we investigated the activity of clockophagy in testes and Sertoli cells. Unexpectedly, clockophagy activity was found to be enhanced by the significantly reduced expression levels of ARNTL and HIF-1α following CPF and TCP exposure. Notably, Arntl knockdown impaired Sertoli cell junctional function. Collectively, these findings strongly indicate that CPF induces ferroptosis in Sertoli cells through activating clockophagy, resulting in the decreased expression of HIF-1α and BTB-associated proteins; this ultimately leads to the disruption of BTB integrity and spermatogenesis dysfunction.
Keywords: Blood-testis barrier; CPF; Clockophagy; Ferroptosis; Sertoli cells.
© 2025 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltdé.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Kermani M., Dowlati M., Gholami M., et al. A global systematic review, meta-analysis and health risk assessment on the quantity of malathion, diazinon and chlorpyrifos in vegetables. Chemosphere. 2021;270 - PubMed
-
- Jing Q., Liu J., Chen A., Chen C., Liu J. The spatial-temporal chemical footprint of pesticides in China from 1999 to 2018. Environ Sci Pollut Res Int. 2022;29(50):75539–75549. - PubMed
-
- Slotkin T.A. Guidelines for developmental neurotoxicity and their impact on organophosphate pesticides: a personal view from an academic perspective. Neurotoxicology. 2004;25(4):631–640. - PubMed
-
- Huang X., Cui H., Duan W. Ecotoxicity of chlorpyrifos to aquatic organisms: a review. Ecotoxicol Environ Saf. 2020;200 - PubMed
-
- Kim H.H., Lim Y.W., Yang J.Y., et al. Health risk assessment of exposure to chlorpyrifos and dichlorvos in children at childcare facilities. Sci Total Environ. 2013;444:441–450. - PubMed
LinkOut - more resources
Full Text Sources

