Validating the antiseizure effects of vitexin and related flavone glycosides in zebrafish
- PMID: 40642006
- PMCID: PMC12241147
- DOI: 10.3389/fphar.2025.1628324
Validating the antiseizure effects of vitexin and related flavone glycosides in zebrafish
Abstract
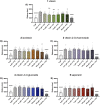
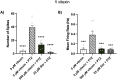
Current epilepsy treatments often fail to provide sufficient control over seizures, highlighting the need for new therapeutic agents. Vitexin, a flavone with antioxidant, anti-inflammatory, and neuroprotective properties, was previously shown to suppress seizure activity in rodent models. Utilizing zebrafish, this study further evaluates the antiseizure properties of vitexin and for the first time, examines the related flavone glycosides: isovitexin, vitexin 2-O-rhamnoside, vitexin-4-O-glucoside and saponarin. We initially tested the ability of the compounds to reduce behavioral seizures stimulated by the GABAA receptor antagonists (pentylenetetrazole: PTZ and picrotoxin: PTX) and spontaneous seizures in a genetic epilepsy model (Dravet syndrome, scn1lab -/- zebrafish larvae). Seizure behavior was quantified in 5-day old larvae via automated tracking with a DanioVision monitoring chamber linked to EthoVision XT 15 software. Microelectrode array electrophysiology (MEA) was then used to examine the effects on PTZ-induced seizure-like brain activity. While having no effect on basal locomotion, vitexin and isovitexin significantly reduced seizure activity in PTZ-treated zebrafish. None of the flavones exhibited antiseizure effects in the PTX-induced epilepsy model. Additional studies with vitexin demonstrated that though it did not suppress spontaneous seizure behaviors in our genetic model of epilepsy, it did significantly inhibit PTZ-induced electrographic activity. These findings support the continued exploration of the translational potential of the vitexin scaffold. This work advances our search for safer, more effective antiseizure drugs and could pave the way for vitexin-based treatments for epilepsy and related disorders.
Keywords: behavior; epilepsy; flavonoids; multielectrode array; vitexin; zebrafish.
Copyright © 2025 Breckenridge, Basnyat, Fitch and Carpenter-Swanson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Yoga for epilepsy.Cochrane Database Syst Rev. 2017 Oct 5;10(10):CD001524. doi: 10.1002/14651858.CD001524.pub3. Cochrane Database Syst Rev. 2017. PMID: 28982217 Free PMC article.
-
Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD001901. doi: 10.1002/14651858.CD001901.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972056 Free PMC article.
-
Treatments for seizures in catamenial (menstrual-related) epilepsy.Cochrane Database Syst Rev. 2021 Sep 16;9(9):CD013225. doi: 10.1002/14651858.CD013225.pub3. Cochrane Database Syst Rev. 2021. PMID: 34528245 Free PMC article.
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2017 Feb 27;2(2):CD001911. doi: 10.1002/14651858.CD001911.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Jul 18;7:CD001911. doi: 10.1002/14651858.CD001911.pub4. PMID: 28240353 Free PMC article. Updated.
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2015 Aug 14;(8):CD001911. doi: 10.1002/14651858.CD001911.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Feb 27;2:CD001911. doi: 10.1002/14651858.CD001911.pub3. PMID: 26275105 Updated.
References
-
- Balamurugam J., Lakshmanan M. (2022). “Screening methods for the evaluation of antiepileptic drugs,” in Introduction to basics of pharmacology and toxicology: volume 3: experimental pharmacology: research methodology and biostatistics. Editors Lakshmanan M., Shewade D. G., Raj G. M. (Singapore: Springer Nature Singapore; ).
LinkOut - more resources
Full Text Sources

