Molecular mechanisms underlying Fagopyrum dibotrys-derived nanovesicles induced ferroptosis in hepatocellular carcinoma: a dual-pathway analysis of lipid peroxidation and mitochondrial damage
- PMID: 40642010
- PMCID: PMC12241094
- DOI: 10.3389/fphar.2025.1636149
Molecular mechanisms underlying Fagopyrum dibotrys-derived nanovesicles induced ferroptosis in hepatocellular carcinoma: a dual-pathway analysis of lipid peroxidation and mitochondrial damage
Abstract
Background: Hepatocellular carcinoma (HCC) is a prevalent malignant tumor globally, with high incidence and mortality rates that seriously endanger human health. While traditional therapeutic approaches have demonstrated some efficacy in controlling disease progression, they are still fraught with numerous limitations. In recent years, plant-derived nanovesicles have garnered significant attention owing to their distinctive biological activities and promising antitumor characteristics. The effects of Fagopyrum dibotrys, a plant with various medicinal values, and its-derived nanovesicles (FdNVs) on HCC cells have not been clarified.
Objective: This study aimed to explore the inhibitory effects of FdNVs on human HCC cells and subcutaneous xenograft tumors, as well as the underlying molecular mechanisms.
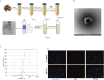
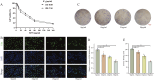
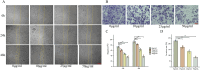
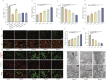
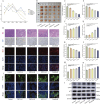
Methods: FdNVs were isolated and purified through ultracentrifugation, characterized via Nanoparticle Tracking Analysis (NTA) and Transmission Electron Microscopy (TEM), and subsequently evaluated in vitro using the HepG2 HCC cell line to assess their effects on proliferation (via cell viability, EdU, and colony formation assays), migration (wound healing assay), and invasion (Transwell assay), while mitochondrial ultrastructural changes were examined by TEM, intracellular ROS and Fe2+ levels were measured fluorometrically, oxidative stress markers (GSH and MDA) were quantified colorimetrically, ferroptosis-related mRNA and protein expression were analyzed by RT-qPCR and Western blot, followed by in vivo validation of their antitumor efficacy in a nude mouse HepG2 xenograft model.
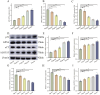
Results: In vitro studies demonstrated that FdNVs dose-dependently suppressed HepG2 cell proliferation, motility, and invasive capacity. Mechanistic investigations revealed that this inhibitory effect was mediated through ferroptosis activation, supported by the following observations: elevated intracellular ferrous iron (Fe2+) and reactive oxygen species (ROS), reduced glutathione (GSH) content, disrupted mitochondrial ultrastructure, and modulated expression of key ferroptosis regulators-including upregulation of pro-ferroptotic proteins (p53 and ALOX15) and downregulation of anti-ferroptotic factors (xCT and GPX4). Furthermore, in vivo studies validated the tumor-suppressive role of FdNVs, confirming their capacity to trigger ferroptosis in HepG2 xenografts.
Conclusion: FdNVs inhibited the proliferation, migration and invasion of HCC cells by inducing iron death, and their anti-tumor mechanism involved the regulation of iron death-related genes and proteins.
Keywords: Fagopyrum dibotrys; ferroptosis; hepatocellular carcinoma; in vitro and in vivo experiments; nanovesicles.
Copyright © 2025 Wu, Chen, Zhang, Tang, Wang, Xue, Gao, Yang and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
[Mechanism of acupuncture on cerebral ischemia-reperfusion injury via p53/SLC7A11/GPX4 signaling pathway in rat models].Zhongguo Zhen Jiu. 2025 Aug 12;45(8):1099-1110. doi: 10.13703/j.0255-2930.20240703-k0003. Epub 2025 May 14. Zhongguo Zhen Jiu. 2025. PMID: 40825694 Chinese.
-
Geniposide Suppresses Tumor Progression Through DUOX1-Mediated Ferroptosis in Hepatocellular Carcinoma.Am J Chin Med. 2025;53(5):1573-1589. doi: 10.1142/S0192415X25500600. Epub 2025 Jul 7. Am J Chin Med. 2025. PMID: 40621626
-
Nobiletin promotes ferroptosis in breast cancer through targeting AKR1C1-mediated ubiquitination and degradation of GPX4.Phytomedicine. 2025 Jul 20;146:157074. doi: 10.1016/j.phymed.2025.157074. Online ahead of print. Phytomedicine. 2025. PMID: 40819642
-
Targeting Ferroptosis With Natural Products to Treat Diabetes and Its Complications: Opportunities and Challenges.Phytother Res. 2025 Jul 9. doi: 10.1002/ptr.70028. Online ahead of print. Phytother Res. 2025. PMID: 40634143 Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

