Drug-associated hearing impairment in children: a disproportionality analysis of the FDA adverse event reporting system
- PMID: 40642015
- PMCID: PMC12241047
- DOI: 10.3389/fphar.2025.1532461
Drug-associated hearing impairment in children: a disproportionality analysis of the FDA adverse event reporting system
Abstract
Objective: Drug-associated hearing impairment has a serious impact on children's quality of life and poses a significant public health burden. However, there is a lack of large-scale population-based studies of medication-associated hearing impairment in children. The aim of this study was to hypothesize about medications through data mining in order to assess the potential risk of these medications increasing hearing impairment in children.
Methods: We extracted and analyzed reports on drugs linked to hearing impairment in children from the FDA Adverse Event Reporting System (FAERS). To assess the relationship between drugs and hearing impairment in children, we performed a disproportionality study utilizing the proportional reporting ratio (PRR) and reporting odds ratio (ROR). Concurrently, we conducted comparisons with medicine labels to identify medications that, although not now indicating hearing impairment in their labels, may possibly pose risks of hearing impairment in children.
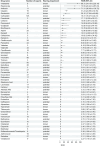
Results: In the FAERS database, there are 1,884 reports of AE related to hearing impairment in children. The top three medications with the highest ROR were vinblastin [N = 6 cases, ROR = 86.72 (34.15-220.19)], risedronate [N = 3 cases, ROR = 73.59 (20.24-267.63)], and amikacin [N = 11 cases, ROR = 71.31 (36.40-139.72)]. The top 3 drugs with the highest number of reports were carboplatin [N = 125 cases, ROR = 18.41 (15.27-22.21)], cisplatin [N = 78 cases, ROR = 31.24 (24.59-39.70)], and vincristine [N = 56 cases, ROR = 6.32 (4.83-8.27)]. Based on drug labeling, 48% drugs (27/56) were classified as potentially ototoxic.
Conclusion: Our findings suggest that nearly half of the 56 drugs linked to hearing impairment signals in children are not currently labeled with ototoxicity warnings. Consequently, further research is required to evaluate the association of these medicines with this risk.
Keywords: FAERS; children; disproportionality; drug-associated hearing impairment; pharmacovigilance.
Copyright © 2025 Liu, Tan, Xiao, Bai, Chang, Su, Wei, Zhong and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Safety evaluation of irinotecan: a real-world disproportionality analysis using FAERS and JADER databases during the time period 2004-2024.Front Pharmacol. 2025 Jun 9;16:1516449. doi: 10.3389/fphar.2025.1516449. eCollection 2025. Front Pharmacol. 2025. PMID: 40552159 Free PMC article.
-
Platinum-induced hearing loss after treatment for childhood cancer.Cochrane Database Syst Rev. 2016 Aug 3;2016(8):CD010181. doi: 10.1002/14651858.CD010181.pub2. Cochrane Database Syst Rev. 2016. PMID: 27486906 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
An exploratory study evaluating the 20 medications most commonly associated with suicidal ideation and self-injurious behavior in the FAERS database.BMC Pharmacol Toxicol. 2025 Jan 30;26(1):24. doi: 10.1186/s40360-025-00858-7. BMC Pharmacol Toxicol. 2025. PMID: 39885564 Free PMC article.
-
A comprehensive, population level evaluation of previously reported drug triggers of pemphigus highlights immunomodulatory capacity as a common characteristic.Front Immunol. 2025 Jan 21;15:1508129. doi: 10.3389/fimmu.2024.1508129. eCollection 2024. Front Immunol. 2025. PMID: 39906745 Free PMC article.
References
-
- Borghi C., Brandolini C., Prandin M. G., Dormi A., Modugno G. C., Pirodda A. (2005). Prevalence of tinnitus in patients withhypertension and the impact of different anti hypertensive drugs on the incidence of tinnitus: a prospective, single-blind, observational study. Curr. Ther. Res. Clin. Exp. 66 (5), 420–432. 10.1016/j.curtheres.2005.10.001 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources