Baicalein from Scutellaria baicalensis mitigates oxidative stress through the IIS pathway in a C. elegans model of ulcerative colitis
- PMID: 40642017
- PMCID: PMC12241015
- DOI: 10.3389/fphar.2025.1592244
Baicalein from Scutellaria baicalensis mitigates oxidative stress through the IIS pathway in a C. elegans model of ulcerative colitis
Abstract
Introduction: Ulcerative colitis (UC) is a chronic, nonspecific inflammatory bowel disease with limited therapeutic options. Baicalein, a phenolic flavonoid extracted from Scutellaria baicalensis, has been traditionally used in Chinese medicine for its potent anti-inflammatory, anti-tumor, and antiviral properties. This plant, known as Huang-Qin, is indigenous to East Asia and has been widely used to treat various conditions such as fever, respiratory diseases, and inflammation.
Aim of the study: This study aimed to establish a C. elegans model of UC induced by dextran sodium sulfate (DSS) and to investigate the protective effects of baicalein on intestinal injury.
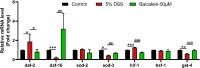
Materials and methods: DSS was used to induce acute intestinal injury in C. elegans. N2 and mutant strains (daf-2 and daf-16) were exposed to DSS at concentrations of 5% (w/v), which identified as optimal for inducing intestinal inflammation. The effects of 25 μM, 50 μM, and 100 μM of baicalein on intestinal barrier function, oxidative stress markers, and relevant gene expression were evaluated, including genes related to epithelial barrier integrity (clc-2, mtm-6, etc.), oxidative stress, and the IIS and p38 MAPK pathways.
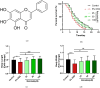
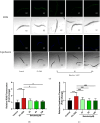
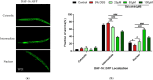
Results: Baicalein significantly improved physiological condition and intestinal permeability in worm treated with 5% DSS. It restored the expression of epithelial barrier genes and reduced oxidative stress, as indicated by decreased ROS, enhancing SOD activity, daf-16 nuclear translocation etc. Baicalein's protective effects were associated with the activation of the p38 MAPK and IIS pathways. In daf-2 and daf-16 mutant strains, baicalein demonstrated partial dependence on the IIS pathway for its protective effects.
Conclusion: This study established a DSS-induced UC model in C. elegans and demonstrated that baicalein exerts protective effects on intestinal barrier integrity and oxidative stress, through the IIS and MAPK pathways. These findings support the use of C. elegans as a model for UC research and provide valuable insights into baicalein's therapeutic potential for inflammatory bowel diseases.
Keywords: C. elegans; IIS pathway; baicalein; dextran sodium sulfate; ulcerative colitis.
Copyright © 2025 Wang, Fu, Xu, Lv, Han, Wang, Xia, Han, Li and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Mechanism of HSP90 Inhibitor in the Treatment of DSS-induced Colitis in Mice by Inhibiting MAPK Pathway and Synergistic Effect of Compound Sophorae Decoction.Curr Pharm Des. 2022;28(42):3456-3468. doi: 10.2174/1381612829666221122113929. Curr Pharm Des. 2022. PMID: 36415092
-
Baicalein ameliorates DSS-induced ulcerative colitis in mice by inhibiting ferroptosis and regulating gut microbiota.Front Pharmacol. 2025 Jul 31;16:1564783. doi: 10.3389/fphar.2025.1564783. eCollection 2025. Front Pharmacol. 2025. PMID: 40822452 Free PMC article.
-
Network pharmacology and experimental validation reveal clotrimazole alleviates dextran sulfate sodium-induced ulcerative colitis by inhibiting intestinal senescence.J Pharmacol Exp Ther. 2025 Jul 18;392(9):103664. doi: 10.1016/j.jpet.2025.103664. Online ahead of print. J Pharmacol Exp Ther. 2025. PMID: 40840121
-
The impact of biological interventions for ulcerative colitis on health-related quality of life.Cochrane Database Syst Rev. 2015 Sep 22;2015(9):CD008655. doi: 10.1002/14651858.CD008655.pub3. Cochrane Database Syst Rev. 2015. PMID: 26393522 Free PMC article.
-
Cyclosporine A for induction of remission in severe ulcerative colitis.Cochrane Database Syst Rev. 2005 Jan 25;(1):CD004277. doi: 10.1002/14651858.CD004277.pub2. Cochrane Database Syst Rev. 2005. PMID: 15674937
References
-
- Althagafy H. S., Ali F. E. M., Hassanein E. H. M., Mohammedsaleh Z. M., Kotb El-Sayed M. I., Atwa A. M., et al. (2023). Canagliflozin ameliorates ulcerative colitis via regulation of TLR4/MAPK/NF-κB and Nrf2/PPAR-γ/SIRT1 signaling pathways. Eur. J. Pharmacol. 960, 176166. 10.1016/j.ejphar.2023.176166 - DOI - PubMed
-
- Ayoub I. M., Eldahshan O. A., Roxo M., Zhang S., Wink M., Singab A. N. B. (2024). Stress resistance, antiaging, and neuroprotective activities of baicalein 5,6-dimethyl ether and Alnus rugosa extract in Caenorhabditis elegans model. Arch. Pharm. Weinh. 357 (12), e2400464. 10.1002/ardp.202400464 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

