Gastrointestinal stromal tumors with the use of ripretinib and sunitinib: real-world adverse event analysis based on the FDA adverse event reporting system (FAERS)
- PMID: 40642018
- PMCID: PMC12242178
- DOI: 10.3389/fphar.2025.1561937
Gastrointestinal stromal tumors with the use of ripretinib and sunitinib: real-world adverse event analysis based on the FDA adverse event reporting system (FAERS)
Erratum in
-
Correction: Gastrointestinal stromal tumors with the use of ripretinib and sunitinib: real-world adverse event analysis based on the FDA adverse event reporting system (FAERS).Front Pharmacol. 2025 Sep 9;16:1689499. doi: 10.3389/fphar.2025.1689499. eCollection 2025. Front Pharmacol. 2025. PMID: 40994637 Free PMC article.
Abstract
Objective: This study aims to analyze potential adverse events (AEs) associated with ripretinib and sunitinb in gastrointestinal stromal tumor (GIST) treatment using data from the FDA Adverse Event Reporting System (FAERS). The findings provide insights for future research to improve the safety and clinical management of ripretinib and sunitinib.
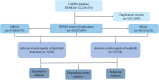
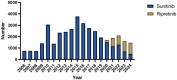
Methods: Adverse Drug Event (ADE) reports related to ripretinib and sunitinib were extracted from the FAERS database, covering the period from Q2 2020 to Q4 2024 and Q1 2006 to Q4 2024, respectively. ADEs were classified and described according to Preferred Terms (PTs) and System Organ Classes (SOCs) in the Medical Dictionary for Regulatory Activities (MedDRA). Disproportionality analysis, including Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-Item Gamma Poisson Shrinker (MGPS), was employed to identify significant signals.
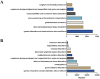
Results: A total of 3,636 and 34,768 ADE reports related to ripretinib and sunitinib were identified using four disproportionality analysis methods. The top five ADR signals for ripretinib include hepatic embolization, tumor compression, hyperkeratosis, tumor excision and tumor pain. For sunitinib, the five strongest ADR signals are metastatic renal cell carcinoma, diffuse uveal melanocytic proliferation, renal cancer metastasis, connective tissue neoplasm and salivary gland fistula. Both drugs share significant ADRs including palmar-plantar erythrodysesthesia syndrome, disease progression and hyperkeratosis. Furthermore, subgroup analysis was conducted to explore sex difference in ripretinib and sunitinib.
Conclusion: This study validated known AEs and identified new potential safety signals associated with ripretinib and sunitinib in GIST treatment. These findings contribute to the understanding of ripretinib and sunitinib, providing valuable evidence for improving its clinical use.
Keywords: FAERS; GIST treatment; adverse events; ripretinib; sunitinib.
Copyright © 2025 Che and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Blay J.-Y., Serrano C., Heinrich M. C., Zalcberg J., Bauer S., Gelderblom H., et al. (2020). Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet, Oncol. 21, 923–934. 10.1016/S1470-2045(20)30168-6 - DOI - PMC - PubMed
-
- Godfrey H., Leibovit-Reiben Z., Jedlowski P., Thiede R. (2025). Alopecia associated with the use of semaglutide and tirzepatide: a disproportionality analysis using the FDA adverse event reporting system (FAERS) from 2022 to 2023. J. Eur. Acad. Dermatol Venereol. JEADV 39, e153–e154. 10.1111/jdv.20197 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

