The influence of H. pylori infection in HER2-positive gastric cancer cell lines: insights from Wnt/β-catenin pathway
- PMID: 40642068
- PMCID: PMC12240786
- DOI: 10.3389/fimmu.2025.1550651
The influence of H. pylori infection in HER2-positive gastric cancer cell lines: insights from Wnt/β-catenin pathway
Abstract
Introduction: The impact of H. pylori infection on the efficacy of trastuzumab in HER2-positive gastric cancer (GC) remains poorly understood, despite growing evidence that tumor microenvironment and host-pathogen interactions influence therapeutic outcomes. This study aimed to investigate how H. pylori strains of differing virulence, one high (HV-HP) and one low (LV-HP), affect GC cell behavior, particularly in the context of ERBB2 (HER2) amplification and Trastuzumab (TRAS)-resistance.
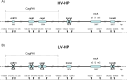
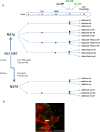
Methods: We used the HER2-amplified NCI-N87 GC cell line, alongside four non-HER2-amplified cell lines (AGS, SNU-1, SNU-16 and SNU-5), to examine the impact of infection. TRAS-resistant derivative cells (N87R) were generated by gradual exposure of the sensitive parental N87 cells (N87p) to increasing TRAS concentrations. Both N87R and N87p cells were infected with HV-HP and LV-HP strains and then treated with epidermal growth factor (EGF), TRAS or a combination of both. The infection was confirmed by confocal microscopy and downstream effects of gene expression were evaluated, focusing on Wnt-β-catenin signaling genes linked to metastasis and survival in HER2+ GC. HER2, PD-L1 and PD-L2 protein levels were assessed in all cell lines using multicolor flow cytometry (FACS) before and after HV-HP exposure.
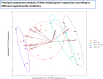
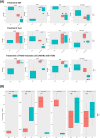
Results: Our data revealed that HV-HP infection reduced MSH6 mRNA expression, which is indicative of impaired DNA repair, and up-regulated PDCD1LG2, suggesting enhanced immunosuppression. FACS analysis showed that HV-HP modulated PD-L2 expression in HER2-amplified N87 cells and to a lesser extent in SNU-16 and SNU-1 cells, while EGF administration increased PD-L1 expression. A strong correlation was observed between ERBB2 expression and TP53, but it was independent of HV-HP. A reduction of CDH1/SNAI ratio was associated with TRAS-resistance in N87 cells.
Discussion: These results suggest that virulent H. pylori in cell lines may contribute to altering tumor phenotype by downregulating the DNA repair machinery, and favouring immune evasion by inducing the expression of immunosuppressive signals, such as PDCD1LG2. Moreover, we found that HER2-targeted therapy may contribute to modulation of CD1/immune pathway. Further studies are warranted to determine whether these effects are common in HER2+ GC in vivo and whether the coexistence of H. pylori infection and TRAS treatment may influence response to immunotherapy.
Keywords: HER2; Helicobacter pylori; MSH6; PD-L1/PD-L2; TP53; Wnt; gastric cancer; trastuzumab.
Copyright © 2025 De Re, Casarotto, Brisotto, Zanussi, De Zorzi, Repetto, Muraro, Spessotto, Baldo, Racanelli, Lenti, Venerito, Fassan, Steffan, Realdon and Cannizzaro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Cancer Today. Available online at: https://gco.iarc.who.int/today/ (Accessed May 5, 2025).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

