Nanolipoprotein particle (NLP) vaccine confers protection against Yersinia pestis aerosol challenge in a BALB/c mouse model
- PMID: 40642079
- PMCID: PMC12241055
- DOI: 10.3389/fimmu.2025.1603710
Nanolipoprotein particle (NLP) vaccine confers protection against Yersinia pestis aerosol challenge in a BALB/c mouse model
Abstract
Introduction: Yersinia pestis is the etiological agent of plague, a disease that remains a concern as demonstrated by recent outbreaks in Madagascar. Infection with Y. pestis results in a rapidly progressing illness that can only be successfully treated with antibiotics given shortly after symptom onset. Live attenuated or whole cell inactivated vaccines confer protection against bubonic plague, but pneumonic plague has been more difficult to prevent. Novel effective subunit vaccine formulations may circumvent some of these shortfalls. Here, we compare the immunogenicity generated by an advanced subunit vaccine (F1V fusion protein) and a nanolipoprotein particle (NLP)-based vaccine.
Methods: The NLP, a high-density lipoprotein mimetic, provides a nanoscale delivery platform for recombinant Y. pestis antigens LcrV (V) and F1. BALB/c mice were immunized via subcutaneous injection twice, three or four weeks apart. Four weeks later, splenocytes and sera were collected for immune profiling, and mice were challenged with aerosolized Y. pestis CO92.
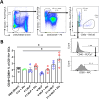
Results: Both formulations induced a strong IgG response against the F1 and V proteins, along with a robust memory B cell response and a balanced cell-mediated immune response as evidenced by both Th1- and Th2-related cytokines. The NLP-based vaccine induced a stronger cytokine response against F1, V, and F1V proteins relative to the F1V vaccine. As with F1V, the inclusion of Alhydrogel (Alu) in NLP vaccine formulations was critical for enhanced immunogenicity and protective efficacy. Mice that received two doses of F1:V:NLP + Alu and CpG were completely protected from a challenge with approximately eight median lethal doses of aerosolized Y. pestis CO92 and this protection confirmed the well-documented synergy between the F1 and V antigens in context of pneumonic plague. The NLPs have defined regions of polarity that facilitates the incorporation of a wide range of adjuvants and antigens with distinct physicochemical properties and are an excellent candidate platform for the development of multi-antigen vaccines.
Keywords: F1; LcrV; Yersinia pestis; mice; nanolipoprotein particle; plague; pneumonic; vaccine.
Copyright © 2025 Biryukov, Rasley, Davies, Klimko, Dankmeyer, Hunter, Rill, Shoe, Miller, Talyansky, Sullinger, Herrera, Huang, Bautista, Pepe, Peters, Xander, Martinez, Toothman, Mlynek, Bozue, Qiu, Fischer and Cote.
Conflict of interest statement
Authors BS, MH, DH, LB and LP were employed by the company Vaxcyte, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Gage KL, Dennis DT, Tsai TF. Prevention of plague; recommendations of the Advisory Committee on Immunization Practices (ACIP). (1996). Available online at: https://www.cdc.gov/mmwr/preview/mmwrhtml/00044836.htm - PubMed
-
- Hinnebusch BJ. Plague in the 21st century: global public health challenges and goals. Natl Institute Allergy Infect Diseases NIH. (2010) 3:87–94.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

