Multi-path direct current spinal stimulation extended survival in the SOD1-G93A model of amyotrophic lateral sclerosis
- PMID: 40642215
- PMCID: PMC12241670
- DOI: 10.3389/fneur.2025.1594169
Multi-path direct current spinal stimulation extended survival in the SOD1-G93A model of amyotrophic lateral sclerosis
Abstract
Introduction: Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that affects motor neurons in the spinal cord and brain. We have developed a novel non-invasive approach, MultiPath-DCS, which utilizes direct current stimulation at multiple sites along the neural axis to provide simultaneous spinal and peripheral stimulation targeted at the affected limbs. MultiPath-DCS modulates the excitability of spinal cord neurons. This effect is significant for ALS, as motor neuron hyperexcitability is a fundamental characteristic of the disease.
Methods: This study used a transgenic mouse model of ALS (SOD1-G93A). Anodal-MultiPath-DCS was applied with six electrodes: three on the spine (centered on T13 and with an anodal polarity), two on the sciatic nerves (one on each nerve), and one on the abdomen. Mice were divided into two groups (stimulated vs. unstimulated or sham-stimulated). The stimulated animals received stimulation for one hour a day, three times a week, for three weeks. Survival was calculated from the onset of the disease and birth until the animal's endpoint. We also performed various electrophysiological and molecular experiments to uncover the mechanism of action.
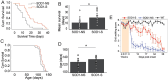
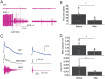
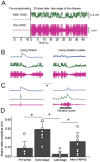
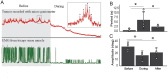
Results: We demonstrated molecular changes induced by anodal MultiPath-DCS, including (a) reduced expression of mutant SOD1 protein, (b) decreased expression of elevated NKCC1, (c) reduced phosphorylated tau, (d) increased expression of HSP70, and (e) increased expression of LC3B. Additionally, we found that treatment with Anodal-MultiPath-DCS (anode on the spinal column) reduces long-term neuronal spinal excitability, slows the progression of muscle weakness, and extends the lifespan of stimulated mice. The mean survival time in the control group was 12.4 days. In comparison, the mean survival time in the stimulated group was 21.6 days using a therapeutic stimulation paradigm, representing a 74% increase in survival from disease onset. Spinal motor neuron survival showed a 54% increase in stimulated compared to non-stimulated groups.
Discussion: Combined, this data provides evidence that Anodal-MultiPath-DCS reduces hyperexcitability and enhances the clearance of misfolded proteins by modulating autophagy and proteolytic systems. By decreasing spinal excitability and clearing toxic proteins from motor neurons, Anodal-MultiPath-DCS promotes survival and could serve as a disease-modifying intervention for ALS.
Keywords: ALS (amyotrophic lateral sclerosis); direct current stimulation (tDCS); neuroprotection; spinal motor neuron; survival.
Copyright © 2025 Ahmed, Samaddar, Hassieb, Sadek, Morozova and Begum.
Conflict of interest statement
ZA holds patents for multi-site neuromodulation and is the scientific founder of PathMaker Neurosystems Inc., a company developing non-invasive devices for treating ALS and stroke based on neuronal hyperexcitability suppression technology directly related to the present work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis.Cochrane Database Syst Rev. 2022 May 20;5(5):CD006981. doi: 10.1002/14651858.CD006981.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593746 Free PMC article.
-
Intranasal Dantrolene Nanoparticles for Treatment of Amyotrophic Lateral Sclerosis as a Disease-Modifying Drug.bioRxiv [Preprint]. 2025 May 27:2025.05.21.655232. doi: 10.1101/2025.05.21.655232. bioRxiv. 2025. PMID: 40501612 Free PMC article. Preprint.
-
Desloratadine alleviates ALS-like pathology in hSOD1G93A mice via targeting 5HTR2A on activated spinal astrocytes.Acta Pharmacol Sin. 2024 May;45(5):926-944. doi: 10.1038/s41401-023-01223-2. Epub 2024 Jan 29. Acta Pharmacol Sin. 2024. PMID: 38286832 Free PMC article.
-
Cdk5 inhibition in the SOD1G93A transgenic mouse model of amyotrophic lateral sclerosis suppresses neurodegeneration and extends survival.J Neurochem. 2024 Sep;168(9):2908-2925. doi: 10.1111/jnc.16160. Epub 2024 Jun 27. J Neurochem. 2024. PMID: 38934222
-
Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2017 Oct 6;10(10):CD004427. doi: 10.1002/14651858.CD004427.pub4. Cochrane Database Syst Rev. 2017. PMID: 28982219 Free PMC article.
References
-
- Salim B, Ahmed Z. Modulation of the sodium/potassium ATPase function and expression by transcranial direct current stimulation of the right sensorimotor cortex in Mice [PhD Thesis]. Academic Works. (2017). Available at: https://academicworks.cuny.edu/gc_etds/2158
LinkOut - more resources
Full Text Sources
Miscellaneous

