Excitation-Dependent Quadruple-Level Emission from an Isolated Molecule for Dynamic Information Encryption
- PMID: 40642888
- PMCID: PMC12520544
- DOI: 10.1002/advs.202508987
Excitation-Dependent Quadruple-Level Emission from an Isolated Molecule for Dynamic Information Encryption
Abstract
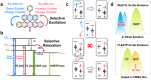
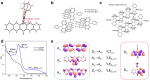
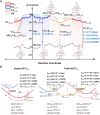
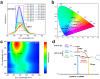
Stimuli-responsive single-molecule multi-emission materials have long attracted considerable attention due to their great potential in non-phase-separated smart luminescence. Here, a new strategy is demonstrated for manipulating electron transfer based on donor-acceptor decoupling to regulate energy levels, aiming to achieve excitation-dependent (Ex-De) single-molecule emission with switchable multiple fluorescence and phosphorescence. The synthesized 10-phenyl-10H,13'H-spiro[acridine 9,6'-pentacen]-13'-one (ACRSP) exhibits anti-Kasha quadruple-level emission and opposite Ex-De afterglow in different environments. The high-energy emission bands of multi-fluorescence in solution respond to excitation, whereas in poly(methyl methacrylate) (PMMA), phosphorescence-fluorescence multi-emission causes Ex-De to appear in the low-energy emission band. Experimental and computational results indicate that exciton spin ratios and emissive state compositions vary with excitation modes, leading to dual Ex-De behavior from three fluorescence and one phosphorescence emissions. Donor-acceptor decoupling separates locally excited (LE) and charge transfer (CT) states, while triplet level inversion enables Ex-De behavior and room-temperature phosphorescence (RTP) coexistence (τ = 770.54 ms). By tuning the excitation mode of ACRSP, we achieve Ex-De long afterglow emission from an isolated molecule, enabling time-resolved and excitation-responsive multi-dimensional information encryption. This work offers design guidelines for purely organic Ex-De systems and paves the way for next-generation single-molecule responsive luminophores.
Keywords: NEVPT2; anti‐kasha emission; excitation‐dependent emission; multi‐dimensional information encryption; room‐temperature phosphorescence.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Yang J., Fang M., Li Z., Acc. Mater. Res. 2021, 2, 644;
- b) Shen Y., Le X., Wu Y., Chen T., Chem. Soc. Rev. 2024, 53, 606. - PubMed
-
- a) Yang Z., Fu Z., Liu H., Wu M., Li N., Wang K., Zhang S.‐T., Zou B., Yang B., Chem. Sci. 2023, 14, 2640; - PMC - PubMed
- b) Huang L., Liu L., Li X., Hu H., Chen M., Yang Q., Ma Z., Jia X., Angew. Chem., Int. Ed. 2019, 58, 16445; - PubMed
- c) Li C., Liu J., Qiu X., Yang X., Huang X., Zhang X., Angew. Chem., Int. Ed. 2023, 62, 202313971; - PubMed
- d) Chen Z., Chen X., Ma D., Mao Z., Zhao J., Chi Z., J. Am. Chem. Soc. 2023, 145, 16748. - PubMed
-
- a) Tian Y., Yang J., Liu Z., Gao M., Li X., Che W., Fang M., Li Z., Angew. Chem., Int. Ed. 2021, 60, 20259; - PubMed
- b) Liu H., Wei S., Qiu H., Si M., Lin G., Lei Z., Lu W., Zhou L., Chen T., Adv. Funct. Mater. 2022, 32, 2108830.
Grants and funding
LinkOut - more resources
Full Text Sources
