TAVI Success Is More Than Just the Valve: CT-Derived Sarcopenia as a Major Determinant of Long-Term Survival
- PMID: 40643039
- PMCID: PMC12246956
- DOI: 10.1002/jcsm.70012
TAVI Success Is More Than Just the Valve: CT-Derived Sarcopenia as a Major Determinant of Long-Term Survival
Abstract
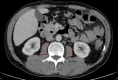
Background: Sarcopenia, characterized by progressive skeletal muscle loss, is a silent yet powerful marker associated with survival, yet its impact on long-term outcomes in transcatheter aortic valve implantation (TAVI) remains underestimated. While frailty has been recognized as a main factor of resilience and recovery, the role of muscle integrity is frequently overlooked. This study explores whether computed tomography (CT)-derived psoas muscle area (PMA) and psoas muscle area index (PMI) are key predictors of post-TAVI survival.
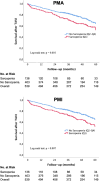
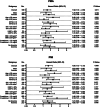
Methods: A total of 539 patients (mean age 82.0 ± 5.1 years; 49.9% female) undergoing TAVI were analysed in this retrospective, single-centre study. Sarcopenia was analysed via sex-specific quartiles of PMA and PMI. Long-term survival was examined using Kaplan-Meier analysis, univariate and multivariate Cox regression analysis. Interaction terms were introduced to assess whether the association between sarcopenia and survival differed by age, sex, renal function and anaemia status.
Results: Sarcopenia emerged as a predictor of long-term mortality (HR = 1.52, p = 0.011 for PMA; HR = 1.55, p = 0.008 for PMI) after TAVI. Notably, younger patients (< 80 years) with sarcopenia faced double the mortality risk (HR = 2.48, p = 0.001 for PMA; HR = 2.55, p = 0.001 for PMI), whereas in older patients, the association was weaker. In women, who typically show a post-TAVI survival advantage, sarcopenia reduced this benefit (HR = 1.75, p = 0.020 for PMA; HR = 1.88, p = 0.008 for PMI). The most striking finding was the synergistic effect of sarcopenia and chronic kidney disease (CKD), resulting in a threefold increase in mortality risk (HR = 2.82, p = 0.027 for PMA; HR 3.14, p = 0.011). After multivariate adjustment, sarcopenia remained a strong, independent predictor of long-term mortality (HR = 1.58, p = 0.009 for PMA; HR = 1.49, p = 0.024 for PMI), reinforcing its clinical relevance in TAVI risk stratification.
Conclusion: Our study suggests that sarcopenia is not just a passive bystander, but may serve as a marker associated with long-term mortality in TAVI patients, especially in younger individuals, women and those with CKD or anaemia. Since muscle mass predicts survival and is potentially modifiable, assessing and intervening against sarcopenia before and after TAVI could represent a clinical priority in patients with aortic valve stenosis. This study underscores the importance of a more nuanced approach-not merely focusing on valve replacement, but on strengthening patient-centred care.
Keywords: TAVI; computed tomography; psoas muscle area; psoas muscle area index; sarcopenia.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
Nikolaos Schörghofer, Christoph Knapitsch, Gretha Hecke, Nikolaus Clodi, Lucas Brandstetter, Crispiana Cozawicz, Matthias Hammerer, Klaus Hergan, Uta C. Hoppe, Bernhard Scharinger and Elke Boxhammer declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Figures

References
-
- Ali S. R., Nkembo A. T., Tipparaju S. M., Ashraf M., and Xuan W., “Sarcopenia: Recent Advances for Detection, Progression, and Metabolic Alterations Along With Therapeutic Targets,” Canadian Journal of Physiology and Pharmacology 102, no. 12 (2024): 697–708, 10.1139/cjpp-2024-0201. - DOI - PMC - PubMed
-
- Bertschi D., Kiss C. M., Schoenenberger A. W., Stuck A. E., and Kressig R. W., “Sarcopenia in Patients Undergoing Transcatheter Aortic Valve Implantation (TAVI): A Systematic Review of the Literature,” Journal of Nutrition, Health & Aging 25, no. 1 (2021): 64–70, 10.1007/s12603-020-1448-7. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

