The Versatility of Diazirines: Properties, Synthetic and Modern Applications
- PMID: 40643049
- PMCID: PMC12319365
- DOI: 10.1002/chem.202500414
The Versatility of Diazirines: Properties, Synthetic and Modern Applications
Abstract
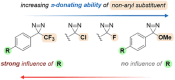
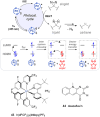
Diazirines are 3-membered heterocycles containing two nitrogen atoms connected by a double bond. They are mostly known for their usage in photoaffinity labeling (PAL), due to their stability and their facile photolysis for on-demand carbene generation. Yet diazirines possess a multi-faceted reactivity that also holds great potential for organic synthesis. This is illustrated in the present review, which is meant to be a beginner's guide for new diazirine users. After briefly summarizing the main synthetic approaches to these derivatives (with a focus on recent improvements), we emphasize some of their most critical features and properties before describing the various modes of activation toward carbene generation, some of which were only uncovered in the past decade. We then review the many modern uses of diazirines, underlining their underappreciated versatility: as carbene precursors in synthesis, but also as electrophilic nitrogen donors, as NMR hyperpolarization probes, or as molecular tools in materials science.
Keywords: carbenes; diazirines; organic synthesis; photochemistry; reactivity.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




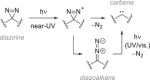




























References
-
- Paulsen S. R., Angew. Chem. 1960, 72, 781.
-
- Schmitz E., Ohme R., Chem. Ber. 1961, 94, 2166.
-
- Graham W. H., J. Am. Chem. Soc. 1962, 84, 1063.
-
- Merritt J. A., Can. J. Phys. 1962, 40, 1683.
-
- Smith R. A. G., Knowles J. R. A., J. Am. Chem. Soc. 1973, 95, 5072. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

