Identification of Transcriptomic Differences in Induced Pluripotent Stem Cells and Neural Progenitors from Amyotrophic Lateral Sclerosis Patients Carrying Different Mutations: A Pilot Study
- PMID: 40643479
- PMCID: PMC12249345
- DOI: 10.3390/cells14130958
Identification of Transcriptomic Differences in Induced Pluripotent Stem Cells and Neural Progenitors from Amyotrophic Lateral Sclerosis Patients Carrying Different Mutations: A Pilot Study
Abstract
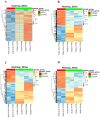
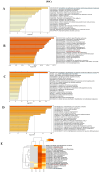
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease affecting motor neurons with a phenotypic and genetic heterogeneity and elusive molecular mechanisms. With the present pilot study, we investigated different genetic mutations (C9orf72, TARDBP, and KIF5A) associated with ALS by generating induced pluripotent stem cells (iPSCs) from peripheral blood of ALS patients and healthy donors. iPSCs showed the typical morphology, expressed stem cell markers both at RNA (OCT4, SOX2, KLF4, and c-Myc) and protein (Oct4, Sox2, SSEA3, and Tra1-60) levels. Moreover, embryoid bodies expressing the three germ-layer markers and neurospheres expressing neural progenitor markers were generated. Importantly, the transcriptomic profiles of iPSCs and neurospheres were analyzed to highlight the differences between ALS patients and healthy controls. Interestingly, the differentially expressed genes (DEGs) shared across all ALS iPSCs are linked to extracellular matrix, highlighting its importance in ALS progression. In contrast, ALS neurospheres displayed widespread deficits in neuronal pathways, although these DEGs were varied among patients, reflecting the disease's heterogeneity. Overall, we generated iPSC lines from ALS patients with diverse genetic backgrounds offering a tool for unravelling the intricate molecular landscape of ALS, paving the way for identifying key pathways implicated in pathogenesis and the disease's phenotypic variability.
Keywords: Amyotrophic lateral sclerosis; RNA-seq; induced pluripotent stem cells; neural progenitor cells; transcriptomic analysis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Frick P., Sellier C., Mackenzie I.R.A., Cheng C.-Y., Tahraoui-Bories J., Martinat C., Pasterkamp R.J., Prudlo J., Edbauer D., Oulad-Abdelghani M., et al. Novel Antibodies Reveal Presynaptic Localization of C9orf72 Protein and Reduced Protein Levels in C9orf72 Mutation Carriers. Acta Neuropathol. Commun. 2018;6:72. doi: 10.1186/s40478-018-0579-0. - DOI - PMC - PubMed
-
- Hulisz D. Amyotrophic Lateral Sclerosis: Disease State Overview. Am. J. Manag. Care. 2018;24:S320–S326. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

