Exosomes as Future Therapeutic Tools and Targets for Corneal Diseases
- PMID: 40643480
- PMCID: PMC12248451
- DOI: 10.3390/cells14130959
Exosomes as Future Therapeutic Tools and Targets for Corneal Diseases
Abstract
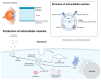
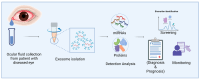
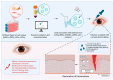
The therapeutic potential of exosomes (Exos), a subpopulation of extracellular vesicles (EVs) secreted by various cell types, has been broadly emphasized. Exos are endosome-derived membrane-bound vesicles 50-150 nm in size. Exos can be general or cell type-specific. Their contents enable them to function as multi-signaling and vectorized vehicles. Exos are important for maintaining cellular homeostasis. They are released into extracellular spaces, leading to uptake by neighboring or distant cells and delivering their contents to modulate cell signaling. Exos influence tissue responses to injury, infection, and disease by fusion with the target cells and transferring their cargo, including cytokines, growth and angiogenic factors, signaling molecules, lipids, DNA, mRNAs, and non-coding RNAs. They are implicated in various physiological and pathological conditions, including ocular surface events, such as corneal scarring, wound healing, and inflammation. Their biocompatibility, stability, low immunogenicity, and easy detectability in bodily fluids (blood, tears, saliva, and urine) make them promising tools for diagnosing and treating ocular diseases. The potential to engineer specific Exo cargos makes them outstanding therapeutic delivery vehicles. The objective of this review is to provide novel insights into the functions of Exo cargos and their applications as biomarkers and therapeutics, or targets in the cornea.
Keywords: biomarkers; cell–cell communication; cornea; crosstalk; exosome blockers; exosomes; miRNAs; therapeutic tools; therapeutics targets.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Dogru M., Chen M., Shimmura S., Tsubota K. Corneal Surgery. 4th ed. Elsevier; Amsterdam, The Netherlands: 2009. Corneal epithelium and stem cells; pp. 25–31.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

