Perioperative Management of Non-Small Cell Lung Cancer in the Era of Immunotherapy
- PMID: 40643492
- PMCID: PMC12248553
- DOI: 10.3390/cells14130971
Perioperative Management of Non-Small Cell Lung Cancer in the Era of Immunotherapy
Abstract
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related mortality worldwide. Nonetheless, deeper molecular understanding of NSCLC has resulted in novel therapeutic approaches, including targeted therapy and immunotherapy, which have improved patient prognosis and outcomes in recent years. Immune checkpoint inhibitors (ICIs), with or without chemotherapy, are now considered valuable components of treatment for NSCLC cases that do not have specific actionable genetic mutations. Patients with actionable genetic mutations are candidates for targeted therapies. The primary focus of this review is the rationale for using ICIs in the perioperative setting for patients with resectable NSCLC and in advanced disease settings. Furthermore, we compare the benefits of using ICIs with the challenges associated with their clinical implementation in resectable and advanced NSCLC. Finally, we emphasize the development of novel treatment strategies that potentially provide an optimal treatment choice for patients with advanced NSCLC.
Keywords: NSCLC; adjuvant therapy; immunotherapy; neoadjuvant therapy; targeted therapy.
Conflict of interest statement
Ilgen Mender is a Director of Biology Research, Sergei M. Gryaznov is the Chief Scientific Officer at MAIA Biotechnology, Inc. Ilgen Mender and Sergei Gryaznov have stock options at MAIA Biotechnology, Inc. The other authors declare no conflicts of interest.
Figures
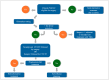
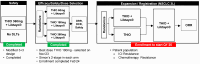
References
-
- Non-Small Cell Lung Cancer Treatment (PDQ®)–Patient Version. [(accessed on 13 June 2025)]; Available online: https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

