From Brain to Blood: Uncovering Potential Therapeutical Targets and Biomarkers for Huntington's Disease Using an Integrative RNA-Seq Analytical Platform (BDASeq®)
- PMID: 40643497
- PMCID: PMC12249049
- DOI: 10.3390/cells14130976
From Brain to Blood: Uncovering Potential Therapeutical Targets and Biomarkers for Huntington's Disease Using an Integrative RNA-Seq Analytical Platform (BDASeq®)
Abstract
Background: Huntington's Disease (HD) remains without disease-modifying treatments, with existing therapies primarily targeting chorea symptoms and offering limited benefits. This study aims to identify druggable genes and potential biomarkers for HD, focusing on using RNA-Seq analysis to uncover molecular targets and improve clinical trial outcomes.
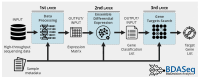
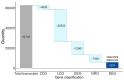
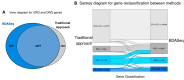
Methods: We reanalyzed transcriptomic data from six independent studies comparing cortex samples of HD patients and healthy controls. The Propensity Score Matching (PSM) algorithm was applied to match cases and controls by age. Differential expression analysis (DEA) coupled with machine learning algorithms were coupled to identify differentially expressed genes (DEGs) and potential biomarkers in HD.
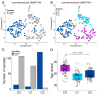
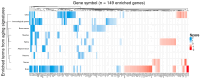
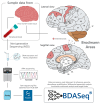
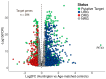
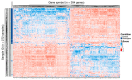
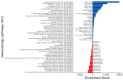
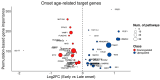
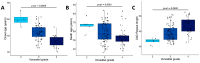
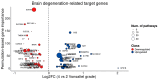
Results: Our analysis identified 5834 DEGs, including 394 putative druggable genes involved in processes like neuroinflammation, metal ion dysregulation, and blood-brain barrier dysfunction. These genes' expression levels correlated with CAG repeat length, disease onset, and progression. We also identified FTH1 as a promising biomarker for HD, with its expression downregulated in the prefrontal cortex and upregulated in peripheral blood in a CAG repeat-dependent manner.
Conclusions: This study highlights the potential of FTH1 as both a biomarker and a therapeutic target for HD. Advanced bioinformatics approaches like RNA-Seq and PSM are crucial for uncovering novel targets in HD, paving the way for better therapeutic interventions and improved clinical trial outcomes. Further validation of FTH1's role is needed to confirm its utility in HD.
Keywords: CAG repeat; FTH1; Huntington’s disease; RNA-Seq; biomarker; blood–brain barrier; druggable genes; neuroinflammation; propensity score matching; transcriptomics.
Conflict of interest statement
The authors J.R.D.P., R.P.A., and B.F.N. declare conflicts of interest with BioDecision AnalyticsTM. J.R.D.P., R.P.A., and B.F.N. are inventors of BDASeq®, a computer program developed and registered in Brazil (registration number BR512025001097-4). Supporting funding obtained for this study was obtained for J.R.D.P., R.P.A., and B.F.N. (BioDecision Analytics Ltd.). J.R.D.P., R.P.A. and B.F.N have been involved as consultants in BioDecision AnalyticsTM.
Figures








Similar articles
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Molecular feature-based classification of retroperitoneal liposarcoma: a prospective cohort study.Elife. 2025 May 23;14:RP100887. doi: 10.7554/eLife.100887. Elife. 2025. PMID: 40407808 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
CD177, MYBL2, and RRM2 Are Potential Biomarkers for Musculoskeletal Infections.Clin Orthop Relat Res. 2025 Jun 1;483(6):1062-1071. doi: 10.1097/CORR.0000000000003402. Epub 2025 Feb 6. Clin Orthop Relat Res. 2025. PMID: 39915095
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
AI-Enhanced Transcriptomic Discovery of Druggable Targets and Repurposed Therapies for Huntington's Disease.Brain Sci. 2025 Aug 14;15(8):865. doi: 10.3390/brainsci15080865. Brain Sci. 2025. PMID: 40867198 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

