Role of Nitric Oxide in Cardioprotection by Poloxamer 188
- PMID: 40643522
- PMCID: PMC12248624
- DOI: 10.3390/cells14131001
Role of Nitric Oxide in Cardioprotection by Poloxamer 188
Abstract
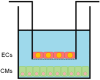
Poloxamer (P) 188 attenuates myocardial ischemia/reperfusion injury through cell membrane stabilization. Cell-cell interactions between endothelial cells (ECs) and cardiomyocytes (CMs) further protect CMs: co-cultures showed that, at an optimal density, ECs protected CMs against hypoxia/reoxygenation (HR) injury. The mechanism of interaction with P188 still requires exploration. We examined if N(ω)-nitro-L-arginine methyl ester (LNAME), a non-specific nitric oxide (NO) synthase inhibitor, abolishes protection in the presence or absence of P188 and/or ECs. We co-cultured mouse coronary artery ECs in an insert atop mouse CMs plated at confluency on the bottom of a well. Normoxic controls remained in complete media while HR groups were exposed to 24 h hypoxia at 0.01% O2 in serum- and glucose-free media, followed by 2 h reoxygenation in complete media. P188 (300 μM), LNAME (40 mM), or vehicle were administered upon reoxygenation. ECs at the used lower density did not decrease HR-triggered lactate dehydrogenase release or calcium overload in CMs by themselves. P188 reduced both indicators after HR by 16/18% without and by 22/25% with ECs, respectively. LNAME abrogated CM protection by P188. Neither intervention had an effect under normoxia. Our co-culture data indicates that P188 requires NO, not necessarily of endothelial origin, to elicit CM protection.
Keywords: NO; P188; calcium; cardiomyocyte; co-culture; co-polymer; cross-talk; endothelium; hypoxia reoxygenation; lactate dehydrogenase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

