Porcine Single-Eye Retinal Pigment Epithelium Cell Culture for Barrier and Polarity Studies
- PMID: 40643526
- PMCID: PMC12248934
- DOI: 10.3390/cells14131007
Porcine Single-Eye Retinal Pigment Epithelium Cell Culture for Barrier and Polarity Studies
Abstract
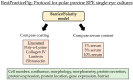
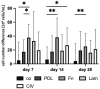
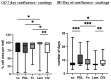
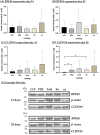
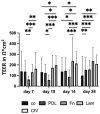
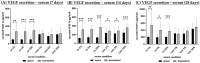
Age-related macular degeneration (AMD) is the main cause of blindness in Western nations. AMD models addressing specific pathological pathways are desired. Through this study, a best-practice protocol for polarized porcine single-eye retinal pigment epithelium (RPE) preparation for AMD-relevant models of RPE barrier and polarity is established. Single-eye porcine primary RPE cells (from one eye for one well) were prepared in 12-well plates including Transwell inserts. Different coatings (laminin (Lam), Poly-ᴅ-Lysine (PDL), fibronectin (Fn) and collagens) and varying serum contents (1%, 5% and 10%) were investigated to determine optimal culture parameters for this model. Success rates of cultures, cell number (trypan-blue exclusion assay), morphology/morphometry (light and fluorescence microscopy), protein secretion/expression (ELISA, Western blot), gene expression (qPCR), transepithelial electric resistance (TEER) and polar location of bestrophin 1 (BEST1) by cryosectioning (IHC-Fr) were assessed. Cells seeded on Lam exhibited the highest level of epithelial cells and confluence properties. Fn resulted in the highest cell number growth. Lam and Fn exhibited the highest culture success rates. TEER values and vascular endothelial growth factor secretion were highest when Lam was used. For the first time, polar (Transwell) porcine single-eye RPE morphometry parameters were determined. RPE on Lam showed bigger cells with a higher variety of cell shapes. CIV displayed the lowest claudin 19 expression. The highest basolateral expression of BEST1 was achieved with Lam coating. The higher the serum, the better the cell number increase and confluence success. A reduction in serum on Lam showed positive results for RPE morphology, while morphometry remained stable. A five percent serum on Lam showed the highest culture success rate and best barrier properties. RPE65 expression was reduced by using 10% serum. Altogether, the most suitable coating of Transwell inserts was Lam, and a reduction in serum to 5% is recommended, as well as a cultivation time of 28 days. A protocol for the use of polar porcine single-eye cultures with validated parameters was established and is provided herein.
Keywords: 3R principle; Transwell; age-related macular degeneration; barrier; best practice protocol; polarity; primary cell culture; retinal pigment epithelium; single-eye culture; tight junctions.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures













References
-
- Wong W.L., Su X., Li X., Cheung C.M.G., Klein R., Cheng C.-Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106-16. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

