The Pathophysiological Role of Vascular Smooth Muscle Cells in Abdominal Aortic Aneurysm
- PMID: 40643529
- PMCID: PMC12248496
- DOI: 10.3390/cells14131009
The Pathophysiological Role of Vascular Smooth Muscle Cells in Abdominal Aortic Aneurysm
Abstract
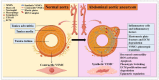
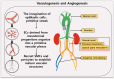
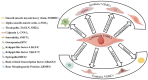
Abdominal aortic aneurysm (AAA) is the most common aortic disease occurring below the renal arteries, caused by multiple etiologies. Currently, no effective drug treatment exists, and the specific pathogenesis remains unclear. Due to its insidious onset and diagnostic challenges, AAA often culminates in aortic rupture, which has a high mortality rate. During AAA development, vascular smooth muscle cells (VSMCs) undergo significant pathological alterations, including contractile dysfunction, phenotypic modulation, cellular degradation, and heightened inflammatory and oxidative stress responses. In particular, emerging evidence implicates vascular smooth muscle cell (VSMC) metabolic dysregulation and mitochondrial dysfunction as key contributors to AAA progression. In this review, we systematically summarize the current understanding of VSMC biology, including their developmental origins, structural characteristics, and functional roles in aortic wall homeostasis, along with the regulatory networks governing the VSMC phenotype and functional maintenance. This review highlights the urgent need for further investigation into the aortic wall VSMC pathophysiology to identify novel therapeutic targets for AAA. These insights may pave the way for innovative treatment strategies in aortic disease management.
Keywords: abdominal aortic aneurysm; aortic lesion; pathophysiology; vascular biology; vascular smooth muscle cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

