Hyperalgesia in the Psychological Stress-Induced Fibromyalgia Model Shows Sexual Dimorphism Mediated by LPA1 and LPA3
- PMID: 40643543
- PMCID: PMC12248790
- DOI: 10.3390/cells14131022
Hyperalgesia in the Psychological Stress-Induced Fibromyalgia Model Shows Sexual Dimorphism Mediated by LPA1 and LPA3
Abstract
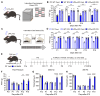
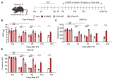
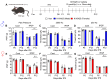
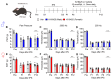
Since the initial report indicating that LPA1 signaling plays a key role in initiating nerve injury-induced neuropathic pain (NeuP), subsequent studies using knockout mice and LPA1/3 antagonists have demonstrated that LPA1 and LPA3 signaling impact NeuP and fibromyalgia (FM) models. In the present study, we identified hyperalgesia sexual dimorphism involving LPA1/3 signaling in the intermittent psychological stress induced-related FM-like model called intermittent psychological stress (IPS)-induced generalized pain (IPGP) model where the hyperalgesia in IPGP mice was abolished in LPA1- and LPA3-knock-out mice. Pharmacological intervention by intraperitoneal (i.p.) treatments with the LPA1/3 antagonist Ki16425 consistently prevented hyperalgesia. However, intracerebroventricular treatments with Ki16425 abolished hyperalgesia in male, but not female, mice. Notably, intrathecal treatments of Ki16425 did not prevent hyperalgesia. Further studies revealed that splenocytes derived from female IPGP mice could initiate hyperalgesia via adoptive transfer in naïve mice, and this effect was abolished when donor mice were pre-treated with Ki16425 (i.p.). Thus, these studies identify male-specific LPA1/3-mediated mechanisms in the brain underlying IPGP, as well as distinct LPA-LPA1/3-mediated peripheral immune mechanisms.
Keywords: Ki16425; LPA1; LPA3; Neurometer; clodronate liposome; empathy; fibromyalgia; knock-out mouse; psychological stress; splenocytes.
Conflict of interest statement
Author Jerold Chun has an employment relationship with Neurocrine Biosciences, a company that may potentially benefit from the research results. Jerold Chun’s relationship with Neurocrine Biosciences has been reviewed and approved by Sanford Burnham Prebys Medical Discovery Institute in accordance with its Conflict of Interest Policies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Tsang A., Von Korff M., Lee S., Alonso J., Karam E., Angermeyer M.C., Borges G.L., Bromet E.J., Demytteneare K., de Girolamo G., et al. Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J. Pain. 2008;9:883–891. doi: 10.1016/j.jpain.2008.05.005. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

