Duchenne Muscular Dystrophy Patient iPSCs-Derived Skeletal Muscle Organoids Exhibit a Developmental Delay in Myogenic Progenitor Maturation
- PMID: 40643552
- PMCID: PMC12249143
- DOI: 10.3390/cells14131033
Duchenne Muscular Dystrophy Patient iPSCs-Derived Skeletal Muscle Organoids Exhibit a Developmental Delay in Myogenic Progenitor Maturation
Abstract
Background: Duchenne muscular dystrophy (DMD), which affects 1 in 3500 to 5000 newborn boys worldwide, is characterized by progressive skeletal muscle weakness and degeneration. The reduced muscle regeneration capacity presented by patients is associated with increased fibrosis. Satellite cells (SCs) are skeletal muscle stem cells that play an important role in adult muscle maintenance and regeneration. The absence or mutation of dystrophin in DMD is hypothesized to impair SC asymmetric division, leading to cell cycle arrest.
Methods: To overcome the limited availability of biopsies from DMD patients, we used our 3D skeletal muscle organoid (SMO) system, which delivers a stable population of myogenic progenitors (MPs) in dormant, activated, and committed stages, to perform SMO cultures using three DMD patient-derived iPSC lines.
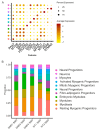
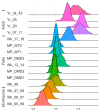
Results: The results of scRNA-seq analysis of three DMD SMO cultures versus two healthy, non-isogenic, SMO cultures indicate reduced MP populations with constant activation and differentiation, trending toward embryonic and immature myotubes. Mapping our data onto the human myogenic reference atlas, together with primary SC scRNA-seq data, indicated a more immature developmental stage of DMD organoid-derived MPs. DMD fibro-adipogenic progenitors (FAPs) appear to be activated in SMOs.
Conclusions: Our organoid system provides a promising model for studying muscular dystrophies in vitro, especially in the case of early developmental onset, and a methodology for overcoming the bottleneck of limited patient material for skeletal muscle disease modeling.
Keywords: Duchenne muscular dystrophy; FAPs; human-induced pluripotent stem cells; myogenesis; myogenic progenitors; organoids; satellite cells; scRNA-seq; skeletal muscle.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Huml R.A. Muscular Dystrophy. Volume 31. Springer; Cham, Switzerland: 2015. Muscular Dystrophy: Historical Background and Types; pp. 5–7. - DOI
-
- Med D.D.-A.G. Undefined Recherches Sur La Paralysie Musculaire Pseudo Hypertrophique Ou Paralysie Myo-Sclerosique. P. Asselin; Paris, Frence: 1868.
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

