Ultrastructural Changes of the Peri-Tumoral Collagen Fibers and Fibrils Array in Different Stages of Mammary Cancer Progression
- PMID: 40643556
- PMCID: PMC12248761
- DOI: 10.3390/cells14131037
Ultrastructural Changes of the Peri-Tumoral Collagen Fibers and Fibrils Array in Different Stages of Mammary Cancer Progression
Abstract
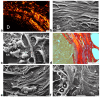
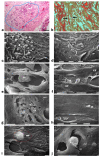
Breast cancer invasion and subsequent metastasis to distant tissues occur when cancer cells lose cell-cell contact, develop a migrating phenotype, and invade the basement membrane (BM) and the extracellular matrix (ECM) to penetrate blood and lymphatic vessels. The identification of the mechanisms which induce the development from a ductal carcinoma in situ (DCIS) to a minimally invasive breast carcinoma (MIBC) is an emerging area of research in understanding tumor invasion and metastatic potential. To investigate the progression from DCIS to MIBC, we analyzed peritumoral collagen architecture using correlative scanning electron microscopy (SEM) on histological sections from human biopsies. In DCIS, the peritumoral collagen organizes into concentric lamellae ('circular fibers') parallel to the ducts. Within each lamella, type I collagen fibrils align in parallel, while neighboring lamellae show orthogonal fiber orientation. The concentric lamellar arrangement of collagen may physically constrain cancer cell migration, explaining the lack of visible tumor cell invasion into the peritumoral ECM in DCIS. A lamellar dissociation or the development of small inter fiber gaps allowed isolated breast cancer cell invasion and exosomes infiltration in the DCIS microenvironment. The radially arranged fibers observed in the peri-tumoral microenvironment of MIBC biopsies develop from a bending of the circular fibers of DCIS and drive a collective cancer cell invasion associated with an intense immune cell infiltrate. Type I collagen fibrils represent the peri-tumoral nano-environment which can play a mechanical role in regulating the development from DCIS to MIBC. Collectively, it is plausible to suggest that the ECM effectors implicated in breast cancer progression released by the interplay between cancer, stromal, and/or immune cells, and degrading inter fiber/fibril hydrophilic ECM components of the peritumoral ECM, may serve as key players in promoting the dissociation of the concentric collagen lamellae.
Keywords: breast cancer; collagen fibers; collagen fibrils; extracellular matrix.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

