Glucocorticoid use in paediatric posterior fossa tumour surgery and the occurrence of postoperative speech impairment
- PMID: 40643729
- PMCID: PMC12254087
- DOI: 10.1007/s00381-025-06850-0
Glucocorticoid use in paediatric posterior fossa tumour surgery and the occurrence of postoperative speech impairment
Abstract
Purpose: Postoperative speech impairment (POSI) is a core symptom of cerebellar mutism syndrome (CMS) and is a common complication after the resection of paediatric posterior fossa (PF) tumours. Preoperative glucocorticoids (pGC) are considered standard treatment to reduce tumour oedema; in addition, glucocorticoids are often administered intraoperatively (iGC) to reduce both postoperative nausea and vomiting. The study aims to investigate whether the occurrence of POSI may be associated with pGC and iGC.
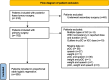
Methods: In a prospective observational multicentre study, we included children with a PF tumour requiring either resection or open biopsy. The use of pGC and iGC, including drug type and dose, was registered. Postoperative speech status was classified as mutism, reduced speech, or habitual speech, where mutism and reduced speech were considered POSI of higher and lower severity, respectively. Proportional odds logistic regression with adjustment for tumour type, tumour location, and age was used to analyse the occurrence of POSI associated with glucocorticoids (GC).
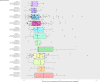
Results: From August 2014 to November 2024, we recruited 810 children, of whom 605 were included in the primary analysis. We found no association between the use of GC (pGC nor iGC) and the occurrence of POSI. The result did not change when adjusting for tumour type, tumour location, and age. The analysis included both a comparison between using and not using pGC (OR 1.06 [95% CI 0.46 -2.49], reference level: use of pGC) and/or iGC (1.28 [0.58-2.82], reference level: use of iGC), and a dose-response analysis of the occurrence of POSI in relation to doubling the dose of GC (pGC OR 1.28 [0.84-1.98]; iGC OR 1.07 [0.62-1.82]).
Conclusion: Our study did not find evidence of a significant change in the occurrence of POSI with the use of pGC or iGC, but our results alone cannot rule out that the administration of pGC or iGC may have some effect. Therefore, our data do not call for a change in recommendations for the use of GC as protection against the development of POSI.
Trial registration number: Clinicaltrials.gov (NCT02300766). Date of registration: November 25, 2014.
Keywords: Brain neoplasm; Cerebellar mutism syndrome; Intraoperative; Neurosurgery; Postoperative speech impairment; Preoperative.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Approved by the Research Ethics Committee of the Capital Region of Denmark (H-6–2014-002). Informed consent: Written informed consent was obtained from the parents. Competing interest: The authors declare no competing interests.
Figures
References
-
- Gudrunardottir T, Morgan AT, Lux AL et al (2016) Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 32(7):1195–1203. 10.1007/s00381-016-3093-3 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 2014-3, 2015-14, 2016-0143, 2018-3653, 2020-6732, 2021-7343/Børnecancerfonden
- 2014-3, 2015-14, 2016-0143, 2018-3653, 2020-6732, 2021-7343/Børnecancerfonden
- 2014-3, 2015-14, 2016-0143, 2018-3653, 2020-6732, 2021-7343/Børnecancerfonden
- 2014-3, 2015-14, 2016-0143, 2018-3653, 2020-6732, 2021-7343/Børnecancerfonden
- 2014-3, 2015-14, 2016-0143, 2018-3653, 2020-6732, 2021-7343/Børnecancerfonden
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous