Computational discovery of potential therapeutic agents against brain-eating amoeba (Naegleria fowleri)
- PMID: 40644400
- PMCID: PMC12250431
- DOI: 10.1371/journal.pone.0327621
Computational discovery of potential therapeutic agents against brain-eating amoeba (Naegleria fowleri)
Abstract
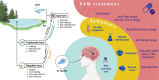
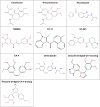
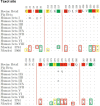
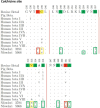
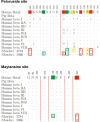
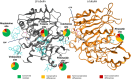
Naegleria fowleri is a human and animal pathogen well-known for its ability to digest neurons and astrocytes of the host's brain, causing a haemorrhagic and necrotizing inflammation called Primary Amoebic Meningoencephalitis. Although infections are rare, the mortality rate is over 97%, due to both the non-specificity of the symptoms and the absence of an effective treatment. In this work we employed bioinformatics tools to evaluate the possibility of treating the infection with tubulin-targeting compounds, which we regard as the most promising approach given the unclear view on the pathogenic factors in N. fowleri, the divergence of the amoeba's tubulins from the human counterparts, and how well-established microtubule-targeting therapies are in clinical practices. The amoeba's tubulin sequences were analyzed and compared to the human tubulins to conjecture the role of their differences in drugs resistance. The binding affinity of the compounds was computed for both species by performing docking simulations using Chemical Computing Group's MOE and CCSB's AutoDock4 and AutoDock Vina. The results were analyzed using a consensus method to increase their reliability. We found that the amoeba's mitotic tubulins show a significant number of changes that are expected to decrease their affinity for tubulin-targeting compounds. We identified the Colchicine binding site as the most suitable target, and propose that Colchicine analogs retain their ability to bind to the amoeba's tubulins in vivo. The selectivity of the compounds for the pathogen however remains an issue. The changes in the amino. acid sequences in the Colchicine site could create a template for designing novel derivatives with an improved selectivity for the parasite and a safer profile for the patient. We therefore believe that our results could be the starting point for a rational derivatization of the selected ligands, leading to the development of an effective treatment for Naegleria fowleri infection.
Copyright: © 2025 Zattoni et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

