Adverse event profile differences among long-acting gonadotropin-releasing hormone analogs: A real-world, pharmacovigilance study
- PMID: 40644420
- PMCID: PMC12250665
- DOI: 10.1371/journal.pone.0327842
Adverse event profile differences among long-acting gonadotropin-releasing hormone analogs: A real-world, pharmacovigilance study
Abstract
Background: Long-acting Gonadotropin-releasing hormone analogs(GnRHa), including leuprolide, goserelin, histrelin, buserelin, triptorelin, have been widely used for a variety of diseases including prostate cancer, breast cancer, endometriosis, uterine leiomyomas, and central precocious puberty (CPP). However, their real-world safety profile differences have not been adequately compared.
Objective: We aimed to investigate the adverse event (AE) profile differences of long-acting GnRHa reported by the US Food and Drug Administration Adverse Event Reporting System (FAERS).
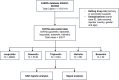
Methods: All indications were searched long-acting GnRHa, as primary suspect drugs, from FAERS data (January 2004 to September 2023). We performed disproportionality analyses by reporting odds ratios (ROR) and conducted univariate and multivariate logistical regression analyses to determine the odds ratio (OR) of serious AEs associated with long-acting GnRHa under different exposure factors.
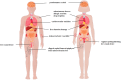
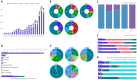
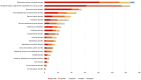
Results: Reproductive system and breast disorders accounted for the greatest proportion of adverse events among the five long-acting GnRHa formulations analyzed. Both buserelin and histrelin showed distinct adverse effect profiles, with buserelin demonstrating a higher incidence of gastrointestinal disorders and histrelin showing a greater propensity for psychiatric disorders. Logistic regression analysis revealed these five medications carried an elevated risk of significant medical events, and this risk was notably lower in pediatric patients (<18 years) compared to adult populations (≥18 years).
Conclusions: Significant disparities exist between the adverse event profiles of long-acting GnRHa. The identification of high-risk factors and the enhancement of AEs monitoring are crucial during clinical application.
Copyright: © 2025 Chen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Del Mastro L, Ceppi M, Poggio F, Bighin C, Peccatori F, Demeestere I, et al. Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: systematic review and meta-analysis of randomized trials. Cancer Treat Rev. 2014;40(5):675–83. doi: 10.1016/j.ctrv.2013.12.001 - DOI - PubMed
-
- Buwenge M, Deodato F, Macchia G, Siepe G, Zhao X, Valicenti RK, et al. Radiotherapy Plus GnRH analogue versus high dose bicalutamide: a case control study. Anticancer Res. 2019;39(11):6373–8. - PubMed
-
- Grigoriadis C, Papaconstantinou E, Mellou A, Hassiakos D, Liapis A, Kondi-Pafiti A. Clinicopathological changes of uterine leiomyomas after GnRH agonist therapy. Clin Exp Obstet Gynecol. 2012;39(2):191–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

