An ingestible device for automated sampling and location tracing in gastrointestinal tract
- PMID: 40644523
- PMCID: PMC12250150
- DOI: 10.1371/journal.pone.0327667
An ingestible device for automated sampling and location tracing in gastrointestinal tract
Abstract
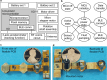
Fluids sampled from the gastrointestinal (GI) tract are of interest for evaluating the bioequivalence of oral medications, and more generally for evaluating GI-related diseases, and for profiling the individual gut microbiome. Existing options for capturing multiple fluid samples from specific locations in the GI tract are limited and invasive, particularly for the small intestine. Here, we report the development of an ingestible capsule for the collection of multiple fluid samples along the GI tract; we additionally report the use of data from sensors within the capsule to determine the sampling regions. The capsule has an ingestible size of Φ14 × 42 mm3. Within this volume, it includes three separate cartridges that capture and retain samples within capillaries; a stepper motor for positioning the sampling cartridges at a sampling port; a 3-axis accelerometer that enables a new method of correlating sample location; a microcontroller with wireless communication and sensor data storage capabilities; and batteries to power the device. We describe in vitro characterization and in vivo tests performed with canine models that have successfully verified the capabilities of the capsule. Fluid samples from the stomach, small intestine, and colon regions of the GI tract are identified by inertial measurements taken within the capsule, and correlated to measurements of the concentration of mesalamine (a drug used for testing) and the bile salt profile in each region, respectively.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Yu A, Baker JR, Fioritto AF, Wang Y, Luo R, Li S, et al. Measurement of in vivo gastrointestinal release and dissolution of three locally acting mesalamine formulations in regions of the human gastrointestinal tract. Mol Pharm. 2017;14(2):345–58. doi: 10.1021/acs.molpharmaceut.6b00641 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

