Exogenous activation of the adhesion GPCR ADGRD1/GPR133 protects against bone loss by negatively regulating osteoclastogenesis
- PMID: 40644539
- PMCID: PMC12248284
- DOI: 10.1126/sciadv.ads3829
Exogenous activation of the adhesion GPCR ADGRD1/GPR133 protects against bone loss by negatively regulating osteoclastogenesis
Abstract
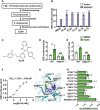
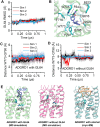
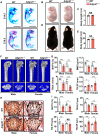
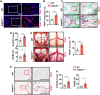
Adhesion G protein-coupled receptors (GPCRs) play crucial roles in numerous physiological and pathological conditions. However, the functions of adhesion GPCRs remain poorly understood because of the lack of effective modulators. Here, we used the adhesion GPCR D1 (ADGRD1/GPR133) as a model to unveil a strategy for finding exogenous agonists that target adhesion GPCRs while revealing previously unidentified functions of ADGRD1. We identified the small molecule GL64 as a selective agonist of ADGRD1. GL64 activates ADGRD1 by mimicking the stachel sequence. Using GL64 as a chemical tool, we demonstrated that ADGRD1 negatively regulates bone loss by inhibiting osteoclastogenesis. The cAMP-PKA-NFATC1 pathway was identified as the downstream signaling pathway of ADGRD1 in osteoclasts. Furthermore, administering GL64 prevented bone loss and suppressed osteoclast activity in the osteoporosis mouse model induced by ovariectomy. Our findings provide mechanistic insights into the activation of adhesion GPCRs by exogenous agonists and underscore the therapeutic potential of targeting ADGRD1 in osteoclast-related diseases.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

