Dynamically covalent lipid nanoparticles mediate CRISPR-Cas9 genome editing against choroidal neovascularization in mice
- PMID: 40644543
- PMCID: PMC12248289
- DOI: 10.1126/sciadv.adj0006
Dynamically covalent lipid nanoparticles mediate CRISPR-Cas9 genome editing against choroidal neovascularization in mice
Abstract
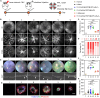
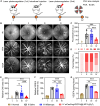
As an important modality for choroidal neovascularization (CNV) treatment, intravitreal injection of vascular endothelial growth factor A (VEGFA) inhibitors suffers from undesired response rate, low patient compliance, and ocular damage. Here, dynamically covalent lipid nanoparticles (LNPs) were engineered to mediate VEGFA gene editing and CNV treatment by codelivering Cas9 mRNA (mCas9) and single guide RNA (sgRNA) targeting VEGFA (sgVEGFA). A library of lipidoids bearing iminoboronate ester linkage was developed via facile "one-pot" synthesis, and the top-performing lipidoid-A4B3C7 was formulated into LNP-A4B3C7 with the highest mRNA transfection efficiency. Inside the diseased retinal pigment epithelial cells, LNPs were dissociated upon H2O2-triggered lipidoid degradation, facilitating mRNA/sgRNA release to potentiate the gene editing efficiency. In laser-induced CNV mice, mCas9/sgVEGFA@LNP-A4B3C7 after single intravitreal injection led to pronounced VEGFA disruption and CNV area reduction, outperforming the clinical anti-VEGF drug in eliciting sustained therapeutic effect. This study establishes a robust nonviral platform for mRNA delivery and genome editing and renders a promising strategy for CNV treatment.
Figures







References
-
- Yim J., Chopra R., Spitz T., Winkens J., Obika A., Kelly C., Askham H., Lukic M., Huemer J., Fasler K., Moraes G., Meyer C., Wilson M., Dixon J., Hughes C., Rees G., Khaw P. T., Karthikesalingam A., King D., Hassabis D., Suleyman M., Back T., Ledsam J. R., Keane P. A., De Fauw J., Predicting conversion to wet age-related macular degeneration using deep learning. Nat. Med. 26, 892–899 (2020). - PubMed
-
- Wang S. V., Kulldorff M., Poor S., Rice D. S., Banks A., Li N., Lii J., Gagne J. J., Screening medications for association with progression to wet age-related macular degeneration. Ophthalmology 128, 248–255 (2021). - PubMed
-
- Doyle S. L., Ozaki E., Brennan K., Humphries M. M., Mulfaul K., Keaney J., Kenna P. F., Maminishkis A., Kiang A. S., Saunders S. P., Hams E., Lavelle E. C., Gardiner C., Fallon P. G., Adamson P., Humphries P., Campbell M., IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Sci. Transl. Med. 6, 230–241 (2014). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

