Association of Medication Use and 8-Year Mortality Risk in Patients With Parkinson Disease: Drug-Wide Trial Emulation
- PMID: 40644656
- PMCID: PMC12264975
- DOI: 10.1212/WNL.0000000000213783
Association of Medication Use and 8-Year Mortality Risk in Patients With Parkinson Disease: Drug-Wide Trial Emulation
Abstract
Background and objectives: There are currently no treatments that can halt or slow the progression of Parkinson disease (PD). The aim of this study was to identify new drug repurposing candidates for PD among existing prescription drugs that could be used to modify the disease course.
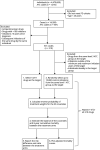
Methods: This nationwide observational cohort study (2004-2020) used Norwegian health registries and was conducted as a high-throughput drug screen using an emulated target trial design. All individuals who met our prescription-based classification criteria for PD, were older than 25 years at the time of diagnosis, and were not prescribed the target drug in the past 2 years were included. We emulated a target trial for any drug filled by a minimum of 100 individuals at any pharmacy in Norway, which amounted to a total of 219 drugs. Mortality was used as an outcome to indicate disease progression. We estimated the effect of drug initiation, an observational analog of the intention-to-treat effect, on the 8-year risk of death, comparing initiators of the target drug with initiators of drugs within the same Anatomical Therapeutic Chemical classification system level 1 group. Inverse probability of treatment weighting was used to adjust for potential confounders.
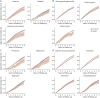
Results: The study included 14,289 individuals with PD (mean age 72 at diagnosis, 59% male) and identified 23 drugs associated with reduced mortality risk at 8 years. These drugs included ranitidine (histamine-2 blocker); pantoprazole and esomeprazole (proton pump inhibitors); losartan (angiotensin receptor blocker); atorvastatin (for high cholesterol); tadalafil (for erectile dysfunction); levothyroxine sodium (thyroid hormone); phenoxymethylpenicillin, erythromycin, and azithromycin (antibiotics); 4 nonsteroidal anti-inflammatory drugs; combined codeine/paracetamol and tramadol (opioid analgesics); zopiclone and melatonin (sleep aids); mianserin (antidepressant); mometasone (nasal corticosteroid); 2 opium-derived cough medicines; and dexamethasone (ophthalmologic corticosteroid).
Discussion: Our study identified several drugs with potential disease-modifying properties that could be candidates for future clinical trials. It highlights the potential of repurposing existing medications to advance drug development. While these findings are exploratory and, therefore, insufficient to justify immediate clinical application, they warrant further investigation and potential inclusion in clinical trials.
Conflict of interest statement
C.R. Scherzer's work is supported by NIH grants National Institute of Neurological Disorders and Stroke/National Institute on Aging R01NS115144, the US Department of Defense, the American PD Association Yale Center for Advanced Parkinson Research, the Rosenbaum PD Drug Development Program, the Lineberger Research Fund, and the Stephen & Denise Adams Center for Parkinson's Disease Research of Yale School of Medicine. All other authors report no relevant disclosures. Go to
Figures
Comment in
-
It Is Time for Drug Repurposing in Parkinson Disease.Neurology. 2025 Aug 12;105(3):e213972. doi: 10.1212/WNL.0000000000213972. Epub 2025 Jul 11. Neurology. 2025. PMID: 40644655 No abstract available.
References
-
- Hernan MA, Robins JM. Causal Inference: What if. Chapman & Hall/CRC; 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
