Factors related to blood-based biomarkers for neurodegenerative diseases and their intergenerational associations in the Young Finns Study: a cohort study
- PMID: 40645733
- PMCID: PMC12242518
- DOI: 10.1016/j.lanhl.2025.100717
Factors related to blood-based biomarkers for neurodegenerative diseases and their intergenerational associations in the Young Finns Study: a cohort study
Abstract
Background: Blood-based biomarkers (BBM) of neurodegenerative diseases are emerging as cost-effective tools in the differential diagnostics of Alzheimer's disease and other dementias. Scarce data exist about factors explaining BBM variation in population-based cohorts, and their intergenerational associations are unknown. This study aimed to characterise BBM distributions among a population-based cohort, investigate the association of a wide array of factors with BBM both in midlife and old age, and investigate intergenerational associations of BBM.
Methods: We measured BBM detecting amyloid β and tau pathologies, including amyloid β42, amyloid β40, and phosphorylated Tau (pTau)-217, as well as glial fibrillary acidic protein (GFAP) and neurofilament light chain (NfL) in the multigenerational Young Finns Study participants (n=1237, age 41-56 years) and their parents (n=814, age 59-90 years) using the Quanterix Simoa HD-X analyser. Standard statistical methods were used to examine the associations between BBM and age, sex, genetic factors, a plethora of cardiometabolic markers, liver and kidney function, and lifestyle factors, as well as their intergenerational associations.
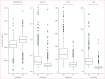
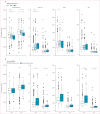
Findings: Increased age was associated with adverse BBM concentrations. Of the various investigated factors, the most robust associations towards adverse BBM concentration were found for APOE ε4 carrier status among parents (amyloid β42:40 ratio, pTau-217, and GFAP) and high serum creatinine concentration in both generations (pTau-217, GFAP, and NfL). Several factors related to glucose metabolism and dyslipidaemia were negatively associated with all BBM, but adjusting for BMI diluted many of these associations. Statistically significant intergenerational correlations ranged from 0·20 to 0·33 and were mostly observed between mothers and offspring in pTau-217, GFAP, and NfL. No intergenerational correlations existed in amyloid β42:40 ratio.
Interpretation: We identified several factors that might influence BBM concentrations, parental transmission being one of them. For reliable use of BBM in clinical practice, it is important to identify which factors directly link to amyloid β and tau pathology and which factors influence BBM concentrations due to other physiological processes.
Funding: Research Council of Finland, Social Insurance Institution of Finland, Competitive State Research Financing of the Expert Responsibility area of the Kuopio, Tampere and Turku University Hospitals, Juho Vainio Foundation, Paavo Nurmi Foundation, Finnish Foundation for Cardiovascular Research, Finnish Cultural Foundation, The Sigrid Juselius Foundation, Tampere Tuberculosis Foundation, Emil Aaltonen Foundation, Yrjö Jahnsson Foundation, Signe and Ane Gyllenberg Foundation, Jenny and Antti Wihuri Foundation, Diabetes Research Foundation of the Finnish Diabetes Association, EU Horizon 2020, European Research Council, Tampere University Hospital Supporting Foundation, Finnish Society of Clinical Chemistry, the Jane and Aatos Erkko Foundation, and the Finnish Brain Foundation.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interest MJ has received lecture fees from Amgen, AstraZeneca, Boehringer Ingelheim, NovoNordisk, and Novartis. EJ is a board member of the Finnish Foundation for Cardiovascular Research. H-MP has received payment and travel support from Roche Diagnostics for a short presentation in EuroMedLab 2023. HZ has served at scientific advisory boards and/or as a consultant for AbbVie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Quanterix, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, and has given lectures sponsored by Alzecure, BioArctic, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, Roche, and WebMD. HZ is Chair of the Alzheimer’s Association Global Biomarker Standardization Consortium and Chair of the IFCC WG-BND. KB has served as a consultant, at advisory boards, or at data monitoring committees for Abbvie, AC Immune, ALZpath, Aribio, Beckman Coulter, BioArctic, Biogen, Eisai, Neurimmune, Ono Pharma, Sanofi, Julius Clinical, Lilly, Novartis, Prothena, Roche Diagnostics, and Siemens Healthineers. HZ and KB are cofounders of Brain Biomarker Solutions in Gothenburg, which is a part of the GU Ventures Incubator Program. All other authors declare no competing interests.
Figures

References
-
- Hunter TR, Santos LE, Tovar-Moll F, De Felice FG. Alzheimer’s disease biomarkers and their current use in clinical research and practice. Mol Psychiatry. 2025;30:272–284. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

