Parkinson's paradox: alpha-synuclein's selective strike on SNc dopamine neurons over VTA
- PMID: 40646031
- PMCID: PMC12254511
- DOI: 10.1038/s41531-025-01055-3
Parkinson's paradox: alpha-synuclein's selective strike on SNc dopamine neurons over VTA
Abstract
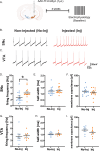
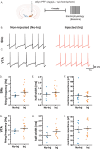
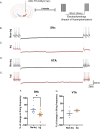
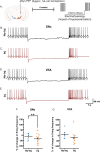
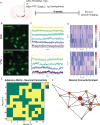
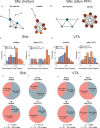
A central question in Parkinson's disease (PD) and related synucleinopathies research is why dopamine neurons in the substantia nigra pars compacta (SNc) are more vulnerable than those in the ventral tegmental area (VTA). We investigated how α-synuclein affects neuronal activity before cell death using two mouse models: α-synuclein preformed fibril injections and AAV-mediated human α-synuclein expression. Four-weeks post-injection, histological analysis confirmed no significant neuronal loss in either structure, providing a temporal window to study neuronal activity before cell death. Electrophysiological recordings revealed region-specific vulnerability: SNc dopamine neurons exhibited significantly increased baseline firing rates while VTA neurons remained unaffected. SNc neurons showed impaired homeostatic firing regulation following hyperpolarization, while VTA neurons maintained normal recovery. Elevated α-synuclein also altered network stability in SNc dopamine neurons before cell death, while sparing VTA neurons. These findings reveal early functional differences that may explain the selective vulnerability of SNc dopamine neurons in PD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Update of
-
Parkinson's Paradox: Alpha-synuclein's Selective Strike on SNc Dopamine Neurons over VTA.bioRxiv [Preprint]. 2025 Apr 2:2025.03.24.644952. doi: 10.1101/2025.03.24.644952. bioRxiv. 2025. Update in: NPJ Parkinsons Dis. 2025 Jul 11;11(1):207. doi: 10.1038/s41531-025-01055-3. PMID: 40236072 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

