Evaluating breast cancer screening performance without registries using medico-administrative data
- PMID: 40646076
- PMCID: PMC12254203
- DOI: 10.1038/s41598-025-10115-w
Evaluating breast cancer screening performance without registries using medico-administrative data
Abstract
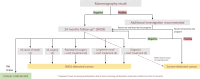
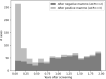
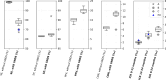
The French Breast Cancer Screening Program (DOCS) was created to detect early Breast Cancer (BC). Key performance indicators for digital mammography include sensitivity (SE), positive predictive value (PPV), interval cancer rate (ICR) and cancer detection rate (CDR). Calculating these metrics requires a linkage between screening data and BC registries; however, registries are scarce in France and often inaccessible for research. We therefore used medico-administrative data as an alternative. We linked regional screening data to the French National Health Data System (SNDS) between 2011 and 2020. Women were followed for 24 months post-screening. Screen-detected cancers and those identified with the SNDS were included. Performance metrics were calculated based on these linked datasets. A total of 252,786 screening exams were analyzed, covering 29,661-33,447 screenings annually, with a mean age of 61 years. SE was 77.9% (95% CI 76.3-79.3), indicating that approximately four in five cancers were detected through mammography. PPV was 19.8% (95% CI 19-20.5), meaning that one in five women with a positive screening test were confirmed with cancer within 24 months. CDR was 10.9 per 1000 exams (95% CI 10.5-11.3), equating to one detected case per 100 screenings. ICR was 2.4 per 1000 exams (95% CI 2.2-2.6), meaning that more than two interval cancers were detected per 1000 screenings. This identification approach using medico-administrative data offers a reproducible alternative for regions where cancer registries are unavailable. A future study applying this methodology in a registry-covered region could further validate the effectiveness of linking screenings to SNDS data for systematic cancer identification.
Keywords: Cancer registries; Data linkage; European health policy; Full-field digital mammography (FFDM); Interval cancer rate; Organized breast cancer screening; Performance assessment; SNDS (National Health Data System) administrative data.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: All screened women within the scope of this study received either individual or collective information letters asking for their Informed consent before data inclusion. The data linkage of the study as well as all experimental protocols were approved by the CNIL (French national data protection authority) in 2019.
Figures

References
-
- International Agency for Research on Cancer, WHO. Global cancer observatory. https://gco.iarc.fr/. Accessed: 2024-01-29.
-
- Ligue contre le Cancer website, Breast cancer section. https://www.ligue-cancer.net/questce-que-le-cancer/les-types-de-cancer/c..., Accessed: 2024/05/07.
-
- Haute Autorité de Santé. Cancer du sein: quel dépistage selon vos facteurs de risque ? (Technical report, Haute Autorité de Santé, 2014).
-
- Peter, C. Gøtzsche (International agency for research on cancer (IARC) handbooks of cancer prevention, Breast cancer screening, 2002).
-
- Tabár, L., Vitak, B., Chen, T.H., Yen, A.M., Cohen, A. Tot, T., Chiu, S.Y. Chen, S.L., Fann, J.C., Rosell, J., Fohlin, H., Smith, R.A., & Duffy, S.W. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology260(3), 658 (2011). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

