Hyaluronidase: structure, mechanism of action, diseases and therapeutic targets
- PMID: 40646377
- PMCID: PMC12254123
- DOI: 10.1186/s43556-025-00299-y
Hyaluronidase: structure, mechanism of action, diseases and therapeutic targets
Abstract
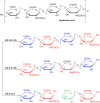
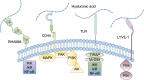
Hyaluronidase (HAase), a family of enzymes critical for regulating physiological and pathological states, catalyzes the degradation of hyaluronic acid (HA), a key component of the extracellular matrix (ECM). By modulating ECM composition and cellular signaling pathways, HAase plays a pivotal role in diverse biological processes, including wound healing, tissue regeneration, and tumor progression. This review systematically elucidates the classification, biological sources, structural diversity, and catalytic mechanisms of HAase, emphasizing its dynamic involvement in disease pathogenesis and diagnostic potential. Furthermore, the article explores innovative therapeutic strategies centered on HAase modulation. HAase inhibitors emerge as promising tools for maintaining HA homeostasis, with implications in anti-inflammatory, antimicrobial, and antitumor therapies by blocking excessive HA degradation. Concurrently, HAase-mediated drug delivery systems represent a paradigm shift in overcoming biological barriers, enhancing bioavailability, and optimizing therapeutic outcomes through ECM remodeling. Notably, the synergy between HAase and immunotherapeutic modalities, such as checkpoint inhibitors and adoptive cell therapies, demonstrates synergistic antitumor effects by reshaping the tumor microenvironment (TME) and augmenting immune cell infiltration. Nevertheless, numerous challenges persist in the clinical application of hyaluronidase, including its immunogenicity, safety, application limitations and ethical considerations. This review synthesizes current research advances and unresolved issues, integrating molecular insights with translational perspectives, aiming to provide a more comprehensive and in-depth understanding of hyaluronidase and to advance clinical therapeutic strategies for hyaluronidase.
Keywords: Extracellular matrix; Hyaluronic acid; Hyaluronidase; Tumor immunotherapy; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: All authors state that there are no potential competing interests.
Figures



Similar articles
-
An oncolytic vaccinia virus encoding hyaluronidase reshapes the extracellular matrix to enhance cancer chemotherapy and immunotherapy.J Immunother Cancer. 2024 Mar 7;12(3):e008431. doi: 10.1136/jitc-2023-008431. J Immunother Cancer. 2024. PMID: 38458640 Free PMC article.
-
Hyaluronidase for reducing perineal trauma.Cochrane Database Syst Rev. 2024 Nov 14;11(11):CD010441. doi: 10.1002/14651858.CD010441.pub3. Cochrane Database Syst Rev. 2024. PMID: 39540564
-
Enzyme cascade-induced optical sensing of hyaluronidase using an ultraviolet-modified liquid crystal interface.Mikrochim Acta. 2025 Sep 3;192(10):628. doi: 10.1007/s00604-025-07499-x. Mikrochim Acta. 2025. PMID: 40897895
-
Extracellular matrix-degradable polymer nanostimulants elicit potent immune responses in orthotopic pancreatic cancer via sono-activatable dual-drug synergism.Mater Today Bio. 2025 Jun 6;33:101954. doi: 10.1016/j.mtbio.2025.101954. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40538751 Free PMC article.
-
Extracellular matrix dynamics in tumor immunoregulation: from tumor microenvironment to immunotherapy.J Hematol Oncol. 2025 Jun 19;18(1):65. doi: 10.1186/s13045-025-01717-y. J Hematol Oncol. 2025. PMID: 40537775 Free PMC article. Review.
References
-
- Sindelar M, Jilkova J, Kubala L, Velebny V, Turkova K. Hyaluronidases and hyaluronate lyases: From humans to bacteriophages. Colloids Surf B Biointerfaces. 2021;208: 112095. 10.1016/j.colsurfb.2021.112095. - PubMed
-
- Lombard V, Bernard T, Rancurel C, Brumer H, Coutinho PM, Henrissat B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem J. 2010;432(3):437–44. 10.1042/BJ20101185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
