Cost-effectiveness analysis of penpulimab plus carboplatin-paclitaxel as first-line treatment for metastatic squamous non-small-cell lung cancer in China
- PMID: 40646431
- PMCID: PMC12255038
- DOI: 10.1186/s13561-025-00656-1
Cost-effectiveness analysis of penpulimab plus carboplatin-paclitaxel as first-line treatment for metastatic squamous non-small-cell lung cancer in China
Abstract
Purpose: Squamous NSCLC (sqNSCLC), a subtype with few targetable mutations, is often diagnosed at advanced stages. Platinum-based chemo-therapy, the first-line treatment, yields median overall survival (OS) of less than or equal to one year, underscoring the need for better therapies. Penpulimab, a novel PD-1 inhibitor developed in China, is a humanized IgG1 antibody with a modified Fc region. Phase III trial data (AK105-302) showed penpulimab + carboplatin-paclitaxel (PEN-CP) significantly improved progression-free survival (PFS) and OS in metastatic sqNSCLC vs. placebo (CP), with a favorable safety profile. However, its high cost and lack of cost-effectiveness analyses warrant further study. This research evaluates PEN-CP's cost-effectiveness vs. CP from the Chinese healthcare perspective.
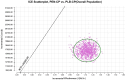
Methods: A three-state Markov model was developed to evaluate the cost-effectiveness of PEN-CP as a first-line treatment for metastatic sqNSCLC. Clinical efficacy data were sourced from the AK105-302 trial, while drug costs were derived from national tender prices. Additional costs and health utilities were obtained from published literature. The primary outcomes included total costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). To assess the robustness of the findings, both one-way and probabilistic sensitivity analyses were conducted.
Results: Compared to CP, the ICER for PEN-CP was $14,918.81 per QALY. The ICER values were below the willingness-to-pay (WTP) threshold of $38,060.00 per QALY. The key drivers of the model outcomes were the price of penpulimab, the PFS stage utility value, and the cost of optimal supportive care.
Conclusions: From the perspective of the Chinese healthcare system, penpulimab combined with first-line chemotherapy demonstrates is cost-effective at a willingness-to-pay threshold of $38,060.00 per QALY for patients with metastatic sqNSCLC and represents a promising first-line treatment option.
Keywords: Carboplatin-paclitaxel; Cost-effectiveness; First-line treatment; Metastatic SqNSCLC; Penpulimab.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN esti-mates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann On-col. 2016;27(suppl 5):v1–27. - PubMed
-
- Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 3.2022: NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497–530. - PubMed
-
- Socinski MA, Obasaju C, Gandara D, et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol. 2018;13(2):165–83. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

