Impact of collaborative pharmaceutical care on older inpatients' medication safety: multicentre stepped-wedge cluster randomised trial (MEDREV Study)
- PMID: 40646458
- PMCID: PMC12247195
- DOI: 10.1186/s12877-025-06122-1
Impact of collaborative pharmaceutical care on older inpatients' medication safety: multicentre stepped-wedge cluster randomised trial (MEDREV Study)
Abstract
Background: Improving medication safety implies patient-centred multidisciplinary cooperation. During the hospital stay for an acute care episode, the patient needs a comprehensive management to guarantee the best possible outcome.
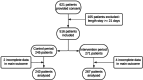
Methods: The study was designed as a non-blinded, multicentre stepped-wedge cluster randomised clinical trial, taking place in six French University Hospitals. Each cluster began with the control period in which standard care did not include pharmaceutical intervention. Every 14-day period, one hospital unit was electronically randomised to switch to the intervention period until all cluster groups received the intervention, which consisted of collaborative pharmaceutical care (CPC) associating medication reconciliation at hospital admission, pharmaceutical analysis of the medication order, medication review and collaborative meeting. The primary outcome was assessing the intervention through the rate of patients with at least one medication error (ME) on the admission medication order (such as omission, wrong dose or wrong route of administration), comparing the two periods.
Results: CPC decreased the rate of patients with at least one ME from 88.9% (n = 243) to 29.2% (n = 267) (p < 0.0001). A total of 1817 MEs were discovered, of which 1121 (61.7%) were in the control period and 696 (38.3%) in the intervention period before resolution by the CPC. After resolving 567 of them, 129 medication errors still remained after CPC. So, a median of 3 MEs [IQR = 1;6] per patient were detected in the control period vs 0 [IQR = 0;1] after CPC in the intervention period (p < 0.0001). Patients were 21-times more likely to avoid a ME with CPC (OR: 20.8 [8.3;52.2], p < 0.0001). The rate of patients with a 2-3 critical ME level decreased from 70.8% to 12.0% in the control vs intervention periods respectively (OR: 18.4 [7.7;43.9], p < 0.0001).
Conclusions: CPC can prevent the occurrence of MEs and thus can improve inpatients' medication management and safety. Pharmacists play a key role in combating medication-related harm in healthcare settings.
Trial registration: This study is registered on ClinicalTrials.gov with the reference number NCT02598115 (2015-11-04).
Keywords: Clinical pharmacy; Collaborative patient management; Geriatrics; Medication error.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics committee approval and consent to participate: The local ethics committee (the Third Southern Mediterranean Protection to Persons Committee) approved the study for all centres (approval number 2015.04.03 bis). All patients included in the study were properly informed regarding their participation and provided written consent at inclusion. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
References
-
- Organization WH. Global patient safety action plan 2021–2030: towards eliminating avoidable harm in health care [Internet]. World Health Organization; 2021 [cited 2025 May 15]. Available from: https://iris.who.int/handle/10665/343477
-
- Alruthea S, Bowman P, Tariq A, Hinchcliff R. Interventions to Enhance Medication Safety in Residential Aged-care Settings: An Umbrella Review. Br J Clin Pharmacol. 2022;88(4):1630–43. - PubMed
-
- Blassmann U, Morath B, Fischer A, Knoth H, Hoppe-Tichy T. Medication safety in hospitals : Integration of clinical pharmacists to reduce drug-related problems in the inpatient setting. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61(9):1103–10. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous