Chronic sleep deprivation induces plasma exosome-derived miR-150-5p downregulation as a novel mechanism involved in Parkinson's disease progression by targeting DCLK1
- PMID: 40646516
- PMCID: PMC12247404
- DOI: 10.1186/s12967-025-06801-y
Chronic sleep deprivation induces plasma exosome-derived miR-150-5p downregulation as a novel mechanism involved in Parkinson's disease progression by targeting DCLK1
Abstract
Background: Researches have suggested that chronic sleep deprivation (SD) can lead to neurological dysfunction and facilitate the onset and progression of Parkinson's disease (PD). However, the association between SD and PD remains unclear. Exosome (exo) cargo comprises microRNAs (miRNAs), which are potential regulators of PD. This study focused on assessing the role and related mechanisms of SD on PD.
Methods: SD plus 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice were used to investigate effects of SD on PD. Exos were extracted from plasma by polymer precipitation method. Impacts of exos on PD were validated through intervention in 1-methyl-4-phenylpyridinium (MPP+)-induced PD cells and MPTP-induced PD mice. Levels of miRNA in exos were analyzed by gene expression profile microarray. Levels of miR-150-5p in exos and substantia nigra pars compacta (SNpc) were further confirmed by reverse transcription quantitative polymerase chain reaction (RT-qPCR). Target genes of miRNAs were predicted by TargetScan and confirmed by Dual-Luciferase Reporter Assay. Mimics and inhibitors of miR-150-5p were transfected into MPP+-induced PD cells, while agomir and antagomir of miR-150-5p were stereotaxic intracranial injected into SNpc of SD + MPTP-induced PD mice, enabling the determination of specific molecular mechanisms affecting PD.
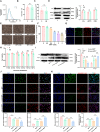
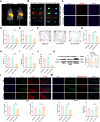
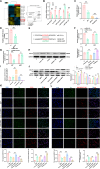
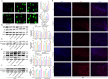
Results: We found that SD and SD-derived exos aggravated PD-related damage. SD-derived exos were identified as potent inducers of PD. MiR-150-5p was recognized as a key element in SD-derived exos, and doublecortin-like kinase 1 (DCLK1) was confirmed as its target gene. Supplementing miR-150-5p alleviated PD damage by inhibiting DCLK1 and abnormal α-synuclein (α-syn) expression, decreasing reactive oxygen species (ROS), p62, cleaved-caspase-3 and cleaved-caspase-9 levels, and increasing Parkin and PINK1 levels and the LC3II/I ratio.
Conclusion: These findings suggested that miR-150-5p-dependent downregulation in SD-derived exos could aggravate the progression of PD via the DCLK1/α-syn pathway. MiR-150-5p decreased ROS levels, promoted mitophagy, and inhibited apoptosis, thus mitigating PD-related damage. These findings indicated that plasma-derived exos and their miRNA cargo might serve as therapeutic targets for PD, providing insights into a mechanism that links SD-related deterioration to the progression of PD.
Keywords: Chronic sleep deprivation; DCLK1; Exosome; Parkinson’s disease; microRNA-150-5p.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments were approved by the Animal Use and Care Committee of Shandong First Medical University and followed national guidelines for animal care and use. Consent for publication: All authors consent for publication. Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–66. - PubMed
-
- Mahul-Mellier AL, Burtscher J, Maharjan N, Weerens L, Croisier M, Kuttler F, Leleu M, Knott GW, Lashuel HA. The process of lewy body formation, rather than simply alpha-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci U S A. 2020;117:4971–82. - PMC - PubMed
-
- Sohrabi T, Mirzaei-Behbahani B, Zadali R, Pirhaghi M, Morozova-Roche LA, Meratan AA. Common mechanisms underlying alpha-Synuclein-Induced mitochondrial dysfunction in Parkinson’s disease. J Mol Biol. 2023;435:167992. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

