An improved immunoassay detects Aβ oligomers in human biofluids: their CSF levels rise with tau and phosphotau levels
- PMID: 40646564
- PMCID: PMC12255133
- DOI: 10.1186/s13195-025-01802-x
An improved immunoassay detects Aβ oligomers in human biofluids: their CSF levels rise with tau and phosphotau levels
Abstract
Background: Diffusible Aβ oligomers (oAβ) confer cytotoxicity in Alzheimer's disease. The dynamic complexity of this hydrophobic analyte means few immunoassays exist to quantify oAβ in CSF and plasma.
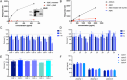
Methods: We characterized antibody 71A1 to a cyclized dimer of Aβ9-18 for oAβ preference over monomers by surface plasmon resonance. We improved an earlier bead-based immunoassay by using 71A1 streptavidin plates for capture and N-terminal antibody 3D6 for detection. Numerous controls systematically validated accuracy.
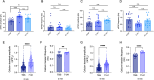
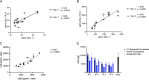
Results: 71A1 showed highly selective binding kinetics to Aβ oligomers over monomers. It enriched bioactive oligomers from AD brain that altered neuronal excitatory currents and calcium transients. 71A1/3D6 immunoassay exhibited specificity and reproducibility in human biofluids. CSF oAβ levels correlated positively with CSF tau and phosphorylated-tau-181. APP and PS1 FAD mutations increased oAβ levels in human neuronal media.
Conclusions: CSF oAβ levels rise in concert with rising tau levels. A new plate-based ELISA offers improved consistency, less sample volume, and lower cost, thus better suited to quantify this challenging analyte.
Keywords: Alzheimer’s disease; Amyloid β-protein; Biomarkers; Monitoring; Oligomeric Aβ; Tau.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All human research subjects received prior approval from the Mass General Brigham Institutional Review Board, and informed consent was obtained for all living human participants. All mouse and rat procedures were approved by the Institutional Animal Care and Use Committee at BWH (IACUC protocol # 2016N000342 and 2016N000305). iPSC lines were utilized following IRB review and approval through MGB/BWH IRB (#2015P001676). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

