Energetic constraints of metal-reducing bacteria as biocatalysts for microbial electrosynthesis
- PMID: 40646565
- PMCID: PMC12247439
- DOI: 10.1186/s13068-025-02666-x
Energetic constraints of metal-reducing bacteria as biocatalysts for microbial electrosynthesis
Abstract
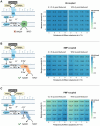
Background: As outlined by the Intergovernmental Panel on Climate Change, we need to approach global net zero CO2 emissions by approximately 2050 to prevent warming beyond 1.5 °C and the associated environmental tipping points. Future microbial electrosynthesis (MES) systems could decrease net CO2 emissions by capturing it from industrial sources. MES is a process where electroactive microorganisms convert the carbon from CO2 and reduction power from a cathode into reduced organic compounds. However, no MES system has attained an efficiency compatible with a financially feasible scale-up. To improve MES efficiency, we need to consider the energetic constraints of extracellular electron uptake (EEU) from an electrode to cytoplasmic electron carriers like NAD+. In many microbes, EEU to the cytoplasm must pass through the respiratory quinone pool (Q-pool). However, electron transfer from the Q-pool to cytoplasmic NAD+ is thermodynamically unfavorable. Here, we model the thermodynamic barrier for Q-pool dependent EEU using the well-characterized bidirectional electron transfer pathway of Shewanella oneidensis, which has NADH dehydrogenases that are energetically coupled to proton-motive force (PMF), sodium-motive force (SMF), or uncoupled. We also tested our hypothesis that Q-pool dependent EEU to NAD+ is ion-motive force (IMF)-limited in S. oneidensis expressing butanediol dehydrogenase (Bdh), a heterologous NADH-dependent enzyme. We assessed membrane potential changes in S. oneidensis + Bdh on a cathode at the single-cell level pre to post injection with acetoin, the substrate of Bdh.
Results: We modeled the Gibbs free energy change for electron transfer from respiratory quinones to NADH under conditions reflecting changes in membrane potential, pH, reactant to product ratio, and energetically coupled IMF. Of the 40 conditions modeled for each method of energetic coupling (PMF, SMF, and uncoupled), none were thermodynamically favorable without PMF or SMF. We also found that membrane potential decreased upon initiation of EEU to NAD+ for S. oneidensis on a cathode.
Conclusions: Our results suggest that Q-pool-dependent EEU is both IMF-dependent and is IMF-limited in a proof-of-concept system. Because microbes that rely on Q-pool-dependent EEU are among the most genetically tractable and metabolically flexible options for MES systems, it is important that we account for this thermodynamic bottleneck in future MES platform designs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Masson-Delmotte V, Zhai P, Pörtner HO, IPCC, et al. IPCC, 2018: global warming of 1.5°C. An IPCC special report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Intergov Panel Clim Change. 2018. 10.1017/9781009157940. - DOI
-
- Zhang S, Jiang J, Wang H, Li F, Hua T, Wang W. A review of microbial electrosynthesis applied to carbon dioxide capture and conversion: the basic principles, electrode materials, and bioproducts. J CO2 Utilization. 2021;51:101640. 10.1016/j.jcou.2021.101640. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
