PARP9-PARP13-PARP14 axis tunes colorectal cancer response to radiotherapy
- PMID: 40646573
- PMCID: PMC12247367
- DOI: 10.1186/s13046-025-03439-y
PARP9-PARP13-PARP14 axis tunes colorectal cancer response to radiotherapy
Abstract
Background: Colorectal cancer (CRC) is the third most prevalent cancer worldwide. Despite substantial advancements in CRC therapy in recent years, ionizing radiation (IR) continues to be the predominant treatment for colon malignances. However, it still lacks the precision required for excellent therapeutic outcomes, ultimately resulting in tumor radioresistance. This study seeks to explore the potential of atypical PARPs including PARP9, PARP12, PARP13 and PARP14 as innovative radiosensitizing targets for CRC.
Methods: We utilized CRISPR/Cas9-mediated gene editing to knockout the PARP9, PARP12, PARP13 and PARP14 in HT29 and DLD1 cells. The cells were exposed to either a single dose of 6-10 Gy or to fractionated dose of 5 × 2 Gy X-ray radiation cultivating cells in 2D, laminin-rich ECM 3D and multicellular spheroid models. The transcriptomes of nonirradiated and irradiated cells were analyzed using microarrays. Gene set enrichment analysis was conducted to determine the pathways in which PARP13 is engaged. Cell viability was assessed using a clonogenic assay. Gene expression levels in cells and patient samples were quantified using RT-qPCR.
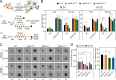
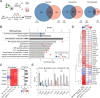
Results: The expression of PARP9, PARP12, PARP13 and PARP14 was particularly elevated in irradiated colorectal cancer HT29 cells in a microenvironment-dependent manner. PARP13 deficiency significantly enhanced the sensitivity of HT29 cells to both single-dose and multifractionated irradiation regimens, resulting in reduced colony formation and spheroidal integrity. Microarray analysis indicated that PARP13 may modulate the expression genes associated with immune response signaling pathways, including members of PARP family. Furthermore, PARP13 loss in HT29 cells markedly impaired the expression of immune response related genes following multifractionated ionizing irradiation. Finally, chemoradiotherapy significantly elevated the expression of PARP9, PARP12, PARP13 and PARP14 in rectal tumors, while having no effect on adjacent normal colon tissues. Elevated pre-treatment PARP9 expression levels and a blunted post-treatment increase in PARP9 and PARP14 expression predicted poor overall survival in rectal cancer patients, while PARP13 emerged as the most significant discriminator between tumor and healthy tissue.
Conclusions: Collectively, the PARP9/13/14 axis is implicated in the response of CRC to radiation treatment in both preclinical and clinical settings, demonstrating the atypical members of the PARP family as attractive targets for neoadjuvant radiotherapy.
Keywords: PARP13; PARP14; PARP9; 3D cell culture; Interferon stimulated genes; Radiosensitizers; Radiotherapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committee of Vilnius Region Biomedical Research (2017-07-04; No. of permission 158200-17-930-433) and informed consent was obtained from all participants. All clinical procedures were conducted at the National Cancer Institute in Lithuania between 2017 and 2022 according to Helsinki regulation. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L et al. Global Cancer Observatory: Cancer Today. 2024. Cancer today. Available from: https://gco.iarc.who.int/today/
-
- Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332(2):237–48. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

