PDGFR-β/Cav1-induced autophagy via mTOR/FIP200/ATG13 activation in cancer-associated fibroblasts promotes the malignant progression of breast cancer
- PMID: 40646615
- PMCID: PMC12247457
- DOI: 10.1186/s12967-025-06831-6
PDGFR-β/Cav1-induced autophagy via mTOR/FIP200/ATG13 activation in cancer-associated fibroblasts promotes the malignant progression of breast cancer
Abstract
Background: Breast cancer incidence rates have been increasing globally. Cancer-associated fibroblasts (CAFs), key stromal components of the tumor microenvironment (TME), play crucial roles in tumor growth by dynamically interacting with cancer cells. Autophagy has been extensively studied in multiple stages of the metastatic cascade. However, the roles of two key membrane proteins, platelet-derived growth factor receptor-β (PDGFR-β) and caveolin-1 (Cav-1), in regulating autophagy in CAFs and their effects on cancer cell invasion and migration remain unclear.
Methods: The association between PDGFR-β expression and clinical features in breast cancer patients was analyzed using TCGA databases. PDGFR-β was either overexpressed or pharmacologically inhibited in cancer cells. Autophagy-related markers and signaling proteins were analyzed by Western blot and RT-qPCR, while lactate secretion and ROS levels were quantified. Breast cancer cell migration and invasion were evaluated through wound healing and transwell assays, and PDGFR-β/Cav1 interactions were verified by immunofluorescence and co-immunoprecipitation (Co-IP). A breast cancer mouse model was employed to assess tumor progression and autophagy modulation in vivo.
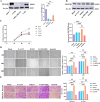
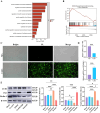
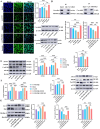
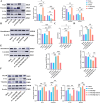
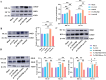
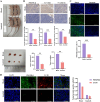
Results: The study demonstrated that PDGFR-β promotes autophagy in CAFs through the mTOR/FIP200/ATG13 signaling. PDGFR-β/Cav-1 enhanced glycolysis in CAFs via autophagy-mediated metabolic reprogramming, resulting in increased lactate export that promoted breast cancer cell growth. Furthermore, CAFs autophagy regulated breast cancer cell invasion and migration via the HIF-1α/MCT4/MCT1 signaling pathway. These findings reveal that PDGFR-β/Cav-1-mediated autophagy in CAFs enhances breast cancer cell invasion, migration, and epithelial-mesenchymal transition (EMT), collectively highlighting the crucial role of CAFs autophagy in facilitating breast cancer progression.
Conclusions: The study elucidates the mechanism by which PDGFR-β/Cav-1 promotes breast cancer progression through autophagy regulation in CAFs, These findings provide a theoretical basis for potential therapeutic method for treating breast cancer.
Keywords: Autophagy; Breast cancer; Cancer-associated fibroblasts; Caveolin-1; PDGFR-β.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: The Nanchang University Animal Care and Use Ethics Committee accepted the protocols used in this work, which adhered to the regulations and guidelines of the Nanchang University Laboratory Animal Center. Consent for publication: All authors have reviewed and approved the final version of the manuscript and agreed to its publication in Journal of Translational Medicine. Competing interests: The authors report no potential conflicts of interest.
Figures

References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

