The Safety of FeedKind Pet® (Methylococcus capsulatus, Bath) as a Cultured Protein Source in the Diet of Adult Dogs and Its Effect on Feed Digestibility, Fecal Microbiome, and Health Status
- PMID: 40646874
- PMCID: PMC12249425
- DOI: 10.3390/ani15131975
The Safety of FeedKind Pet® (Methylococcus capsulatus, Bath) as a Cultured Protein Source in the Diet of Adult Dogs and Its Effect on Feed Digestibility, Fecal Microbiome, and Health Status
Abstract
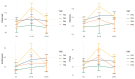
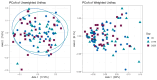
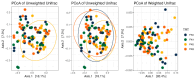
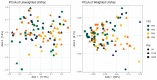
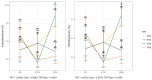
Thirty-two healthy adult dogs (16 males and 16 females) were fed control kibble diets for one month, followed by six months (Weeks 0 to 25) of diets containing either 0, 4, 6, or 8% cultured protein derived from Methylococcus capsulatus (FeedKind Pet®, FK), then they were fed control diets (0% FK) for a further two months (Weeks 25 to 34). The diets were isonitrogenous, isolipidic, and isocaloric and stage- and age-specific. The dogs were assessed for overall health, weight gain, and body condition score (BCS). Blood samples were collected 1 week prior to randomization, during acclimation, then in Weeks 5, 13, 25, 30, 32, and 34 for hematology, coagulation, and clinical chemistry; urine was collected according to the same time schedule for urinalysis. Feces were assessed for parasite load and presence of occult blood during Weeks 5, 9, 13, 17, 21, and 25. Fecal samples were collected during acclimation and Weeks 25 and 34 for fecal microbiome analysis and in Week 25 for apparent total gastrointestinal tract digestibility (ATTD). All dogs maintained a healthy weight and BCS throughout the study. Hematology parameters were within normal limits at the end of each phase of the study. With the exception of a decrease in serum phosphorus level and in urine pH in all groups at the end of the study, urine and serum chemistry results were within normal limits at the end of each phase. ATTD values for organic matter, protein, and energy exceeded 80%, whilst digestibility values for copper were around 20%. The fecal microbiome was dominated by Firmicutes. Alpha diversity increased during the safety phase before returning to baseline levels during the washout phase. The dominant genera in all groups were Megamonas, Peptoclostridium, Turicibacter, Catenibacterium, Fusobacterium, Romboutsia, and Blautia. The study has shown that the inclusion of cultured protein at up to 8% of the total diet of adult dogs can provide sufficient nutrition and is safe with no long-term effects on a range of health parameters.
Keywords: diet formulation; digestibility; microbial protein; microbiome; nutrition; postbiotic; single cell protein; target animal safety.
Conflict of interest statement
B.Q. and K.M. were employed by BSM Partners. W.M. was employed by Charles River Laboratories. The senior author (M.L.) is an employee of Calysta who funded the study and supplied the test ingredient (FeedKind Pet). Although M.L. assisted in the design of the study, this followed FDA and AAFCO guidelines for a target animal safety study with the addition of a 2-month washout period at the end of the 6-month exposure period and additional analysis for digestibility and microbiome. All data collection and analysis was completed without input from M.L. In conjunction with K.M., M.L. wrote the first draft of the manuscript with subsequent editorial input from the other authors.
Figures




References
-
- Alexandratos N., Bruinsma J. World Agriculture Towards 2030/2050: The 2012 Revision. FAO; Rome, Italy: 2012. ESA Working Paper No. 12-03.
-
- Alexander P., Berri A., Moran D., Reay D., Rounsevell M.D.A. The global environmental paw print of pet food. Glob. Environ. Change. 2020;65:102153. doi: 10.1016/j.gloenvcha.2020.102153. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

