From By-Products to Promising Bifunctional Food Ingredients: Physicochemical Characterization and Antioxidant and Emulsifying Improvement Evaluation Based on the Synergy of Phenolic Acids, Flavonoids and Tannins with Bovine Liver Hydrolysates
- PMID: 40646977
- PMCID: PMC12248795
- DOI: 10.3390/foods14132225
From By-Products to Promising Bifunctional Food Ingredients: Physicochemical Characterization and Antioxidant and Emulsifying Improvement Evaluation Based on the Synergy of Phenolic Acids, Flavonoids and Tannins with Bovine Liver Hydrolysates
Abstract
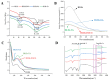
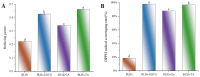
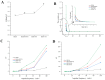
In recent years, bifunctional ingredients extracted and utilized from waste by-products as raw materials have received significant attention in the food production process. Previous studies have found that bovine livers possess both antioxidant and emulsifying potential; therefore, enhancing these dual properties is a current research focus. In this study, three different types of polyphenols (epigallocatechin gallate [EGCG], gallic acid [GA] and tannin [TA]) provide a reference on how to achieve better complexation of polyphenols with bovine liver hydrolysates (BLHs). Based on the molecular weight results, it was shown that the bovine liver peptides bind to polyphenols to form complexes with higher molecular weights. Furthermore, the binding affinities among the three complexes were as follows: TA > EGCG > GA. The emulsions stabilized by the coupling compounds contained more homogeneous and dense droplets (optical microscopy). Both the antioxidant properties and the emulsifying activity of all complexes were superior to those of bovine liver hydrolysates (BLHs) (p < 0.05), confirming synergistic effects that either flavonoids, phenolic acids or tannins possess with bovine liver hydrolysates. This combination provides an effective strategy for developing novel foods with specific functions.
Keywords: binding laws; dual properties; emulsions; microstructure.
Conflict of interest statement
Author Lin Tong was employed by the company Inner Mongolia Horqin Cattle Industry Co. He participated in Software, Investigation, Conceptualization, Methodology, Writing—Review and editing in the study. Author Guangxing Han was employed by the company Han from Shandong Lvyrun Food Co., Ltd. He participated in Software, Investigation, Conceptualization, Methodology, Writing—Review and editing in the study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Trade-offs between selection of crude protein and tannins in growing lambs.J Anim Sci. 2024 Jan 3;102:skae298. doi: 10.1093/jas/skae298. J Anim Sci. 2024. PMID: 39367535
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
References
-
- Zou Y., Shi H.B., Chen X., Xu P.P., Jiang D., Xu W.M., Wang D.Y. Modifying the structure, emulsifying and rheological properties of water-soluble protein from chicken liver by low-frequency ultrasound treatment. Int. J. Biol. Macromol. 2019;139:810–817. doi: 10.1016/j.ijbiomac.2019.08.062. - DOI - PubMed
-
- Agyei D., Ongkudon C.M., Wei C.Y., Chan A.S., Danquah M.K. Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprod. Process. 2016;98:244–256. doi: 10.1016/j.fbp.2016.02.003. - DOI
-
- Liu Q., Kong B.H., Xiong Y.L.L., Xia X.F. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118:403–410. doi: 10.1016/j.foodchem.2009.05.013. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

