Effects of Muscle Fiber Composition on Meat Quality, Flavor Characteristics, and Nutritional Traits in Lamb
- PMID: 40647060
- PMCID: PMC12248750
- DOI: 10.3390/foods14132309
Effects of Muscle Fiber Composition on Meat Quality, Flavor Characteristics, and Nutritional Traits in Lamb
Abstract
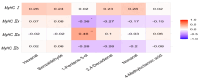
Skeletal muscle fiber type composition critically influences lamb meat quality. This study examined the relationships between muscle fiber types and key quality traits, including tenderness, color, lipid and amino acid profiles, and volatile flavor compounds. MyHC I (slow-twitch oxidative fibers) positively correlated with desirable traits such as increased redness, water-holding capacity, unsaturated fatty acids, and essential amino acids. Conversely, MyHC IIb (fast glycolytic fibers) was linked to reduced tenderness and higher levels of off-flavor compounds. MyHC IIa and IIx showed minimal effects. Untargeted metabolomics comparing muscles with high versus low slow-twitch fiber proportions revealed differential metabolites enriched in sphingolipid and arginine-proline metabolism pathways. These results suggest that a higher proportion of oxidative fibers enhances both the sensory and nutritional qualities of lamb meat by modulating lipid metabolism, amino acid availability, and flavor formation.
Keywords: amino acid; fatty acid; metabolomics; muscle fiber type; volatile flavor compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Van Le H., Nguyen D., Nguyen Q., Malau-Aduli B., Nichols P., Malau-Aduli A. Fatty acid profiles of muscle, liver, heart and kidney of Australian prime lambs fed different polyunsaturated fatty acids enriched pellets in a feedlot system. Sci. Rep. 2019;9:1238. doi: 10.1038/s41598-018-37956-y. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

