Reforming Food, Drug, and Nutraceutical Regulations to Improve Public Health and Reduce Healthcare Costs
- PMID: 40647079
- PMCID: PMC12248432
- DOI: 10.3390/foods14132328
Reforming Food, Drug, and Nutraceutical Regulations to Improve Public Health and Reduce Healthcare Costs
Abstract
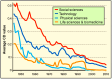
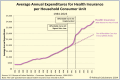
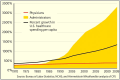
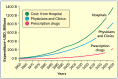
Neglecting preventive healthcare policies has contributed to the global surge in chronic diseases, increased hospitalizations, declining quality of care, and escalating costs. Non-communicable diseases (NCDs)-notably cardiovascular conditions, diabetes, and cancer-consume over 80% of healthcare expenditure and account for more than 60% of global deaths, which are projected to exceed 75% by 2030. Poor diets, sedentary lifestyles, regulatory loopholes, and underfunded public health initiatives are driving this crisis. Compounding the issue are flawed policies, congressional lobbying, and conflicts of interest that prioritize costly, hospital-based, symptom-driven care over identifying and treating to eliminate root causes and disease prevention. Regulatory agencies are failing to deliver their intended functions. For instance, the U.S. Food and Drug Administration's (FDA) broad oversight across drugs, devices, food, and supplements has resulted in inefficiencies, reduced transparency, and public safety risks. This broad mandate has allowed the release of unsafe drugs, food additives, and supplements, contributing to the rising childhood diseases, the burden of chronic illness, and over-medicalization. The author proposes separating oversight responsibilities: transferring authority over food, supplements, and OTC products to a new Food and Nutraceutical Agency (FNA), allowing the FDA to be restructured as the Drug and Device Agency (DDA), to refocus on pharmaceuticals and medical devices. While complete reform requires Congressional action, interim policy shifts are urgently needed to improve public health. Broader structural changes-including overhauling the Affordable Care Act, eliminating waste and fraud, redesigning regulatory and insurance systems, and eliminating intermediaries are essential to reducing costs, improving care, and transforming national and global health outcomes. The information provided herein can serve as a White Paper to help reform health agencies and healthcare systems for greater efficiency and lower costs in the USA and globally.
Keywords: artificial intelligence; health insurance; healthcare cost; hospital sector; innovations; nutrients; nutrition; public health.
Conflict of interest statement
Financial ties between FDA officials and the pharmaceutical industry have undermined public trust [169]. Despite disagreements over fragmented and insufficient evidence, these excessively close associations between FDA and CDC directors and pharmaceutical company sponsors the approval of drugs suggest undue influence in the FDA’s decision process [170,171]. In addition to barring the current too-close associations, it is essential to have stricter policies on post-tenure pharma employment prohibitions, which are necessary for impartiality and to regain the lost public trust [169]. These are essential to restore credibility of the FDA and the trust of the public.
Ensuring transparency in drug approvals remains a critical issue, with concerns about the FDA approving drugs based on borderline results, tolerating protocol violations in clinical trials, and accepting data with inadequate statistical power from pharmaceutical companies [73,158]. Additionally, the denial of drugs that should have been approved due to conflicts of interest within the industry has raised additional ethical concerns [35,172]. Close associations between FDA officials and pharmaceutical sponsors as well as disagreements over insufficient evidence, suggest undue industry influence on the approval process [159,173].
The FDA’s persistent lack of transparency during drug evaluations and refusal to allow independent scientists to review original clinical trial (RCT) data, safety, and efficacy (as with the COVID-19 vaccine) has eroded its credibility and trust [171]. This oversight is crucial for physicians and patients to identify biases and make informed decisions [157]. However, superficial reforms, claims without substantive changes, will not address these issues [174]. The Congressional FDA Reform Act of 2012 failed to eliminate conflicts of interest among FDA employees and government agencies [35,159].
Without strict oversight and accountability, the integrity of the drug and medical device approval process remains compromised, undermining public trust. These external factors, often from drug companies or other stakeholders, exercise inappropriate pressure on the FDA staff to approve a drug or medical device, which could potentially compromise the scientific review process [173], and prioritizes commercial interests over public health concerns [172].
Addressing this issue requires enacting a new Act to eliminate conflicts of interest across government agencies [173]. A major controversy was the approval of COVID-19 mRNA vaccines and antivirals under Emergency Use Authorization despite the availability of cost-effective generic treatments in 2020 [70,71,82,177,178,179,180]. The FDA’s refusal to approve repurposed, effective generic compounds like vitamin D and ivermectin for SARS-CoV-2 [177] led to overwhelmed hospital staff and resources with patients, and unnecessary loss of lives [70,71,72,82]. The DHHS should ensure that such a harmful scenario will not be repeated. Closing regulatory loopholes, eliminating favoritism toward big pharma, and preventing conflicts of interest in the FDA and CDC are crucial [35,172,173].
Cozy relationships between FDA directors and pharmaceutical sponsors and disagreements over insufficient evidence suggest an unwarranted influence on FDA approvals [170,172]. The public considered the non-approval of widely available generics and early therapies against SARS-CoV-2 to be due to conflicts of interest; it had significant negative consequences [71,72]. Policymakers must close these loopholes and enact stronger laws to prevent undue industry influence across the FDA, CDC, and other government agencies [159]. Approvals despite borderline results, tolerating shortcuts in RCTs, and denying economic agents claimed due to ‘insufficient’ evidence further raise concerns of serious conflicts and affect the credibility of the FDA [172]. A new Congressional Act is essential to eliminate conflicts of interest within government agencies [35,170].
The author has no conflicts of interest. He has not received funding, influence, assistance, or professional writing for this perspective policy article.
Figures
References
-
- Ruiz-Nunez B., Pruimboom L., Dijck-Brouwer D.A., Muskiet F.A. Lifestyle and nutritional imbalances associated with Western diseases: Causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. J. Nutr. Biochem. 2013;24:1183–1201. doi: 10.1016/j.jnutbio.2013.02.009. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

