Obesity-Associated NAFLD Coexists with a Chronic Inflammatory Kidney Condition That Is Partially Mitigated by Short-Term Oral Metformin
- PMID: 40647220
- PMCID: PMC12251041
- DOI: 10.3390/nu17132115
Obesity-Associated NAFLD Coexists with a Chronic Inflammatory Kidney Condition That Is Partially Mitigated by Short-Term Oral Metformin
Abstract
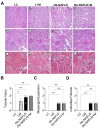
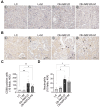
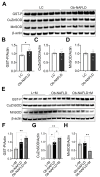
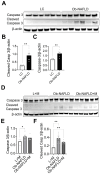
Background/Objectives: Chronic kidney disease (CKD) is twice as prevalent in individuals with obesity-associated non-alcoholic fatty liver disease (Ob-NAFLD), highlighting the need to determine the link and mechanisms of kidney injury as well as explore therapies. Metformin, a first-line treatment for type 2 diabetes, shows promise in managing NAFLD, but its renal benefits in Ob-NAFLD remain unclear. This study investigates the impact of Ob-NAFLD on kidney injury and assesses the potential protective effects of metformin. Methods: Five-week-old female Zucker rats (obese fa/fa and lean Fa/Fa) were fed an AIN-93G diet for 8 weeks to induce Ob-NAFLD, then fed the diet with Metformin for 10 weeks. Kidneys were collected for histopathological and biochemical analyses. Results: Histopathological studies showed increased tubular injury, mesangial matrix expansion, and fibrosis in kidneys with Ob-NAFLD compared to lean control (LC) rats. Immunohistochemistry further revealed an elevated macrophage and neutrophil infiltration and increased levels of nitrotyrosine and p22phox in Ob-NAFLD kidneys. Furthermore, Ob-NAFLD rat kidneys showed upregulation of TNF-α and CCL2 genes and increased levels of caspase-3 (total and cleaved). Interestingly, metformin treatment significantly decreased TNF-α mRNA and blunted nitrotyrosine levels, and modestly reduced immune cell infiltration in Ob-NAFLD. Conclusions: These findings indicate that Ob-NAFLD promotes CKD as evidenced by tubular injury, oxidative stress, inflammation, and fibrosis. While short-term metformin treatment showed anti-oxidative and anti-inflammatory effects in Ob-NAFLD, its impact on structural kidney damage was limited, highlighting the need for longer treatment or alternative therapeutics such as oxidant scavengers and anti-inflammatory drugs to effectively mitigate renal pathologies.
Keywords: inflammation; kidney injury; metformin; obesity-NAFLD; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Bikbov B., Purcell C.A., Levey A.S., Smith M., Abdoli A., Abebe M., Adebayo O.M., Afarideh M., Agarwal S.K., Agudelo-Botero M., et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

