Alcohol Consumption and Liver Metabolism in the Era of MASLD: Integrating Nutritional and Pathophysiological Insights
- PMID: 40647333
- PMCID: PMC12251479
- DOI: 10.3390/nu17132229
Alcohol Consumption and Liver Metabolism in the Era of MASLD: Integrating Nutritional and Pathophysiological Insights
Abstract
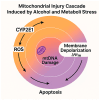
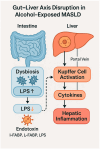
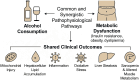
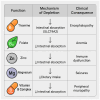
Metabolic dysfunction-associated steatotic liver disease (MASLD) has emerged as the leading cause of chronic liver disease worldwide, driven by the global epidemics of obesity, type 2 diabetes, and metabolic syndrome. In this evolving nosological landscape, alcohol consumption-traditionally excluded from the diagnostic criteria of non-alcoholic fatty liver disease (NAFLD)-has regained central clinical importance. The recently defined MetALD phenotype acknowledges the co-existence of metabolic dysfunction and a significant alcohol intake, highlighting the synergistic nature of their pathogenic interactions. This narrative review provides a comprehensive analysis of the biochemical, mitochondrial, immunometabolic, and nutritional mechanisms through which alcohol exacerbates liver injury in MASLD. Central to this interaction is cytochrome P450 2E1 (CYP2E1), whose induction by both ethanol and insulin resistance enhances oxidative stress, lipid peroxidation, and fibrogenesis. Alcohol also promotes mitochondrial dysfunction, intestinal barrier disruption, and micronutrient depletion, thereby aggravating metabolic and inflammatory derangements. Furthermore, alcohol contributes to sarcopenia and insulin resistance, establishing a bidirectional link between hepatic and muscular impairment. While some observational studies have suggested a cardiometabolic benefit of a moderate alcohol intake, emerging evidence challenges the safety of any threshold in patients with MASLD. Accordingly, current international guidelines recommend alcohol restriction or abstinence in all individuals with steatotic liver disease and metabolic risk. The review concludes by proposing an integrative clinical model and a visual cascade framework for the assessment and management of alcohol consumption in MASLD, integrating counseling, non-invasive fibrosis screening, and personalized lifestyle interventions. Future research should aim to define safe thresholds, validate MetALD-specific biomarkers, and explore the efficacy of multidisciplinary interventions targeting both metabolic and alcohol-related liver injury.
Keywords: CYP2E1; MASLD; MetALD; micronutrient deficiencies; mitochondrial dysfunction; nutritional counseling in liver disease; oxidative stress; sarcopenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



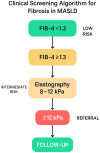
References
-
- Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., Zelber-Sagi S., Wong V.W.-S., Dufour J.-F., Schattenberg J.M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. - DOI - PubMed
-
- Adinolfi L.E., Marrone A., Rinaldi L., Nevola R., Izzi A., Sasso F.C. Metabolic dysfunction-associated steatotic liver disease (MASLD): A systemic disease with a variable natural history and challenging management. Explor. Med. 2025;6:1001281. doi: 10.37349/emed.2025.1001281. - DOI
-
- Le P., Tatar M., Dasarathy S., Alkhouri N., Herman W.H., Taksler G.B., Deshpande A., Ye W., Adekunle O.A., McCullough A., et al. Estimated Burden of Metabolic Dysfunction-Associated Steatotic Liver Disease in US Adults, 2020 to 2050. JAMA Netw. Open. 2025;8:e2454707. doi: 10.1001/jamanetworkopen.2024.54707. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

