EpiSwitch PSE Blood Test Reduces Unnecessary Prostate Biopsies: A Real-World Clinical Utility Study
- PMID: 40647492
- PMCID: PMC12249354
- DOI: 10.3390/cancers17132193
EpiSwitch PSE Blood Test Reduces Unnecessary Prostate Biopsies: A Real-World Clinical Utility Study
Abstract
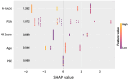
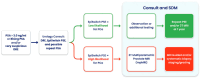
Background/Objectives: Prostate cancer (PCa) remains a major contributor to cancer-related morbidity and mortality worldwide. Current diagnostic strategies, largely based on PSA screening, lack specificity and sensitivity, leading to unnecessary invasive procedures and elevated healthcare costs. This real-world study evaluated the EpiSwitch® PSE assay, a blood-based test analyzing 3D genome conformation signatures, ability to avoid unnecessary biopsies and the resulting clinical and economical benefits. Methods: 187 patients undergoing evaluation for PCa were tested with the EpiSwitch® PSE assay. Biopsy confirmation was available for 53 patients, while predictive modeling assessed 134 patients using EpiSwitch PSE results and clinical variables. Results: Among the 187 patients evaluated, predictive modeling showed that up to 79.1% (106/134) of patients could safely defer biopsy based on a low-likelihood EpiSwitch PSE result, while an alternative model showed a 66.4% (89/134) biopsy avoidance rate. The PSE result demonstrated strong concordance with biopsy-confirmed diagnoses and was the most influential predictor in multivariate analysis, followed by PI-RADS score. The test achieved a 100% technical success rate, with an average turnaround time of 4.4 days. Conclusions: Incorporating the EpiSwitch PSE assay into clinical workflows enhances decision-making efficiency, reduces unnecessary biopsies, and improves healthcare resource utilization. These findings support the assay's strong clinical utility and economic value, highlighting its potential for broader adoption as a minimally invasive reflex test and a pre-biopsy triage tool for the early and accurate detection of prostate cancer. Future studies should include prospective, multicenter trials to confirm these results across broader populations and evaluate longitudinal outcomes of patients managed with PSE-guided care.
Keywords: 3D genome conformation; EpiSwitch PSE assay; biopsy avoidance; blood-based diagnostics; precision oncology; prostate cancer detection; real-world evidence.
Conflict of interest statement
Joe Abdo and Ryan Mathis are full-time paid employees of Oxford BioDynamics Inc. in the USA. Ewan Hunter and Alexandre Akoulitchev are full-time paid employees of Oxford BioDynamics PLC in the UK. Joos Berghausen is a part-time paid intern at Oxford BioDynamics and is a full-time PhD student at Georgetown University Medical Center. Dr. Pohlman is a paid clinical advisor for Oxford BioDynamics and a board-certified practicing urologist at Kearney Urology Center PC. The views expressed in this paper are solely those of the authors and do not necessarily reflect the views, policies, or positions of Oxford BioDynamics. The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

